Abstract
Background
The information on seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among patients with inflammatory bowel disease (IBD) and its comparison to healthy controls is sparse. We compared the seroprevalence rates in patients with IBD and healthy controls (HCs).
Methods
Patients with IBD and HCs (contact of patients) underwent SARS-CoV-2 antibody testing (chemiluminescent immunoassay: Siemens kit IgG against antigen-S1RBD) between July 2020 and April 2021. Information on demography, disease characteristics, drug history and past history of SARS-CoV-2 infection were noted. Patients on 5-aminosalicylic acid or no treatment were considered not on immunosuppressants and those who had received steroids, thiopurines or methotrexate within six months of inclusion were considered being on immunosuppressants.
Results
A total of 235 patients (51.9%, males; mean age, 38.7 ± 12.4 years; median disease duration, 60 months [interquartile range, IQR: 36–120]) (ulcerative colitis [UC]: 69.4%, Crohn’s disease [CD]: 28.9%, IBD unclassified [IBDU]: 1.7%) and 73 HCs (mean age, 39.6 ± 10.9 years, 80% males) were enrolled. Of the 235 patients, 128 (54.5%) patients were on immunosuppressants and 107 (45.5%) were not on immunosuppressants. Seventy-four (31.5%) patients were seropositive, of which two (0.9%) had previous history of SARS-CoV-2 infection and none received coronavirus disease-19 (COVID-19) vaccine. Seroprevalence between IBD patients and HCs (32% vs. 27%, p > 0.05) and between patients with and without immunosuppressants (28.1% vs. 36%, p > 0.05) was similar. Age, gender, disease type, duration and activity in the last six months; and medication use were similar between patients with positive and negative serology. There was a progressive increase in seroprevalence from July 2020 to April 2021.
Conclusion
Up to 1/3rd of patients with IBD were seropositive for immunoglobulin G (IgG) SARS-Cov-2 antibody indicating high seroprevalence in patients with IBD from Northern India.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a ribonucleic acid (RNA) virus first reported with human infection in Wuhan province of China in December 2019 [1]. Since then, the virus has spread rapidly globally causing coronavirus disease - 19 (COVID-19), which continues to have a devastating effect on global health. SARS-CoV-2 infection ranges from asymptomatic to mild infection to severe pneumonia that can result in fatality. It is generally believed that good immunity protects from SARS-CoV-2 infection and disease. Immunosuppression caused by drugs and disease may predispose to SARS-CoV-2 infection or may be associated with worse outcomes. However, the literature on this aspect to date is scarce and the available literature suggests that these groups of patients are neither at increased risk nor at risk of poor outcomes.
Inflammatory bowel disease (IBD) burden is increasing in India and other Asian countries and considering Indian population, the overall disease burden is one of the highest in world [2,3,4]. Immunosuppressive drugs such as corticosteroids, thiopurines, methotrexate and anti-tumor necrosis factor (anti-TNF) agents (tumor necrosis factor agents) are being used in patients of IBD to control the disease activity. These drugs can lead to an immunosuppressive state, which might predispose to increased rates of infection and adverse outcomes when compared to healthy asymptomatic persons in the community [5]. The International Organization for the Study of IBD (IOIBD) had recommended that the patients with IBD need to be cautious and take safety precautions such as wearing a mask and maintaining social distancing [6]. But in the initial days of the pandemic, evidence was lacking in terms of difference in SARS-CoV-2 infection rates between patients with immune-mediated diseases and healthy controls. Humoral immune responses against SARS-CoV-2 proteins, including receptor binding domain (RBD) of spike S protein, induce production of antibodies [7, 8]. Thus, serological positivity to SARS-CoV-2 antibody determines the previous infection to SARS-CoV-2 irrespective of symptoms [9]. Multiple studies from different populations reported seropositivity rates in patients with IBD range from 0.5% to 21% [10,11,12,13,14,15,16,17,18,19,20,21]. However, the data of seroprevalence rates from Asian population, where the IBD disease burden is high, is lacking. Moreover, with the advent of vaccination, the post-vaccination antibody response could interfere with the interpretation of positive serology, especially when being conducted against the spike protein. So, we aimed to study the seroprevalence rates among patients with IBD and compare them with their healthy contacts, especially during the period when vaccination had just begun in India, so as to include only non-vaccinated adults with IBD.
Methodology
Patient population
This study prospectively enrolled outpatients with IBD (both ulcerative colitis [UC] and Crohn’s disease [CD]), attending the IBD clinic between July 2020 and April 2021. Healthy contacts of patients were included as healthy controls (HCs). Adult patients with IBD, age > 18 years and willing to give consent were included. Patients on biologics were excluded from the study. Healthy controls included were devoid of any illness and were not on any immunosuppressive medications at the time of inclusion. Informed consent was obtained from patients and healthy controls. The samples were collected cross-sectionally, with data collection at the time of recruitment. Patients were not followed up subsequently.
Data collection
After informed consent, participants were given a questionnaire, which was separately designed for patients and HCs. The questionnaire for patients included the details of disease and activity (nature of illness, disease duration, disease activity at the enrollment and over past six months), treatment details (nature, dose and duration of treatment in the past six months and any alteration made after the COVID-19 pandemic) and SARS-CoV-2 infection details (history of confirmed COVID-19 in the past, history of symptoms suggestive of COVID-19 in the past six months and the history of exposure to a confirmed or suspected case of COVID-19). Questionnaire for HCs included predominantly about the SARS-CoV-2 infection details similar to patients with IBD.
Ethics clearance
This study was started after the approval of institute ethics committee (IEC: 288/17.04.2020).
Sample collection
After filling the questionnaires, a 5-mL blood sample was withdrawn from the participants. Serum separation of sample was done and sample was stored at − 80 °C. Siemens SARS-CoV-2 immunoglobulin (IgG) assay, which works on the technique chemiluminescence against antigen S1RBD (Siemens Healthcare Diagnostics Inc. 511, Benedict Avenue, Tarrytown, NY 10591, USA) was used for analysis. The cut-off for seropositivity was taken as antibody titer > 1 U/mL.
Definitions used in the study
Inflammatory bowel disease (UC, CD and IBD unclassified: diagnosed as per ECCO [European Crohn’s and Colitis Organization]) guidelines are based on clinical, endoscopic, radiologic and histologic features [22]. Disease extent, location and behavior were classified as per the Montreal classification [23]. Clinical disease activity in UC and CD was measured by the simple clinical colitis activity index (SCCAI) and Crohn’s disease activity index (CDAI), respectively [24, 25].
Patients with IBD were classified as being on immunosuppressants if they were on following medications at inclusion or had received them in the past six months: corticosteroids at a dose more than (prednisolone equivalent) 20 mg/day for > 2 weeks or immunomodulators at any dose (azathioprine/6-mercaptopurine/methotrexate) [5]. Patients who were either on no medication or only on 5-aminosalicylic acid agents were classified as not being on immunosuppressants.
Outcomes of the study
Included determination of SARS-CoV-2 seroprevalence in patients with IBD, comparison with HCs and comparison between patients with and without immunosuppressants.
Statistical methods
Statistical Package for the Social Sciences (SPSS) software (version 26.0, IBM Corp, Armonk, NY, USA) was used for statistical analysis. Categorical variables are expressed in percentages. Normally distributed continuous variables were expressed as mean (standard deviation) and the continuous variables with skewed distribution were expressed as median (interquartile range [IQR]). The Mann–Whitney U test was used for comparison of continuous non-normally distributed variables and the Chi-square or Fisher’s test for discrete variables, wherever applicable. Normally distributed continuous variables were compared with the Student t test. p-value < 0.05 was taken as significant.
Results
Patient population
Total 235 patients with IBD and 73 HCs were included from July 2020 to April 2021. The number of patients and HCs recruited each month is mentioned in Supplementary Table 1. Of 235 patients, 163 (69.4%) had UC, 68 (28.9%) had CD and the rest four (1.7%) had IBD unclassified (Table 1).
Baseline clinical, demographic and laboratory characteristics
The mean age of IBD patients at enrolment was 38.7 ± 12.4 years (UC and IBDU: 36.9 ± 11.5, CD: 42.8 ± 13.8 years). The mean age of HCs (39.6 ± 10.9 years) was comparable to patients with IBD. Fifty-two per cent of IBD patients and 80% of the HCs were males. Median disease duration of patients with IBD was 60 months (IQR: 36–120 months, UC: 60 [36–120], CD: 72 [39–120] months). A majority of patients with UC had left-sided colitis (55%, n = 91) followed by pancolitis (43%, n = 71). Terminal ileal disease (68%, n = 46) and inflammatory behavior (52%, n = 35) were the major phenotypes of patients with CD. More than 50% of the patients with IBD were in remission at inclusion (69%, n = 161) and during the six-month period (55%, n = 130) before enrolment. A few patients (4.2%, n = 10) had comorbidities such as diabetes mellitus, hypertension, coronary artery disease and liver disease (Table 1). None of the HCs had comorbidities.
Treatment details
A majority of patients with IBD and UC were receiving 5-aminosalicylate medications (73.1%, n = 172; 95%, n = 158, respectively), while a majority of patients with CD were on immunomodulators (62%, n = 42) (Table 1). No patient in the study was receiving either anti-TNFs or anti-integrins. No patient received vaccination as this study was started prior to the implementation of the vaccination drive. In the overall cohort, 128 (54.4%) patients were on immunosuppressants and the rest 107 patients (45.6%) were not on immunosuppressants. Only two patients with IBD and none of the HCs had past history of symptomatic COVID-19. None of the patients or HCs reported symptoms suggestive of COVID-19 or contact with COVID-19 patients.
Seroprevalence of SARS-CoV-2 infection
Seropositivity against SARS-CoV-2 was determined after (IgG) assay with a cut-off value of more than 1 U/mL. Seropositivity rates were comparable between patients with IBD and HCs (32% vs. 27%, p > 0.05) as shown in Fig. 1. No significant differences were noted in the seropositivity rates between patients with UC and CD (32% vs. 29%, p > 0.05). On the comparison between seropositive and seronegative patients of IBD, there was no significant difference seen in the terms of age, gender and disease duration, activity and treatment type (Table 2).
Seroprevalence of SARS-CoV-2 infection with timing
To evaluate the effect of timing of sample collection on COVID-19 seropositivity, the analysis was done with respect to three time periods — July to September 2020, October to December 2020 and January to April 2021. Though similar seroprevalence rates were observed between the patients of UC and CD at the three different time periods during the course of the study, there was a gradual increase in seroprevalence with time, increasing from 19% in July to September 2020 to 25% 33% in October to December 2020 to 36% 38% in the time period from January to April 2021 (Fig. 2). However, a reverse trend was observed in HCs, which could be due to low sample size, as only 10 and 23 HCs could be recruited in the second and third time periods, respectively, as compared to 40 during the first time period (Supplementary Table 1).
Comparison between patients with IBD with respect to immunosuppressant use
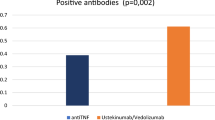
Fifty-four percent (n = 128) of patients were on immunosuppression (49% [n = 82] with UC and 68% [n = 46] with CD). SARS-CoV-2 seroprevalence rates were comparable between patients with IBD with and without immunosuppressant use (28% vs. 36%, p > 0.05) as shown in Fig. 3. Similar results were replicated in patients of UC (33% vs. 32%, p > 0.05), while patients with CD on immunosuppression had lower seroprevalence rates when compared to patients without (20% vs. 50%, p = 0.01). Disease activity over the last six months did not affect the seroprevalence rates in the IBD cohort (Fig. 4). On sub-group analysis among patients on immunosuppressants, the seropositivity among patients on steroids, steroids and immunomodulators (azathioprine/ 6-MP/ methotrexate) and immunomodulators alone was 8/25 (40%), 5/22 (23%), and 23/81 (28.3%), respectively.
Comparison of antibody titers
The median (IQR) antibody titers were also similar between patients with IBD and healthy controls (3.98 [IQR: 1.95–6.51] vs. 3.63 [IQR: 1.91–10.01], p = 0.92) and between patients with and without immunosuppressant use (3.79 [IQR: 1.86–6.93] vs. 4.33 [IQR: 1.93–6.49], p = 0.91).
Discussion
The present study demonstrated a relatively high seroprevalence against SARS-CoV-2 in patients with IBD from Northern India. Approximately one in every three patients with IBD was positive, although only two patients had a documented history of COVID-19. History suggestive of COVID-19 and contact with COVID-19 patient were also not present in a majority of the patients, indicating asymptomatic infection in most of the patients. The patients were recruited between July 2020 and April 2021 and there was a progressive increase in seroprevalence rates from July 2020 to April 2021. The seroprevalence rates were similar between patients with UC and CD and there was no effect of age, gender and disease duration or disease activity on the seroprevalence rates. These rates are much higher when compared with studies in patients with IBD conducted in other parts of the world during the same time period. Most of these studies have been conducted in Western Europe (UK, Italy, Germany, Poland) and the USA (Table 3) and the seroprevalence rates in these studies varied from 0.5% to 21% [10,11,12,13,14,15,16,17,18,19,20,21]. The probable reason for the high seroprevalence rates in our study compared to other global studies might be high infection rates in the community during the same time period. Comparing with other studies from India, conducted during the same time period, the seroprevalence rates in general population varied from 8% to 58% and that in healthcare workers varied from 2.5% to 24%. The progressive rise was also matched with infection rates in the general population during the same time period. In the Indian Council of Medical Research pan Indian seroprevalence studies, there was a progressive increase in seroprevalence rates, being 1% in May to June 2020, 7% in August to September 2020, 23% in December 2020–January 2021 and 67.6% in June–July 2021 [26,27,28,29]. Furthermore, in a recent meta-analysis of 53 studies with 905,379 participants, the pooled seroprevalence rates were 20.7% during the first wave and 69.2% in the second wave in the studies from India [30].
We also observed that immunosuppression did not predispose to increased risk of SARS-CoV-2 infection in patients with IBD. Most patients in studies from other parts of the globe were on biologics such as anti-TNF agents or vedolizumab and a few patients were only on immunomodulators such as thiopurines or only on 5-aminosalicylic acid therapy. Hence, the present study becomes unique in terms of its patient population, as none of these patients was on biologics and unlike other studies, about 50% of patients were not on any immunosuppression. This provided us the opportunity to compare the effect of disease as well as drugs on the risk of infection with SARS-CoV-2 and expectedly, neither the disease nor the drugs affected the seroprevalence rates. The seroprevalence was similar between patients with IBD and healthy controls and the rates were independent of any immunosuppressant drug intake, including steroids, thiopurines or methotrexate. These results are similar to other studies in patients with IBD, which have reported prevalence rates similar to their respective populations, indicating that disease and immunosuppression did not increase the seroprevalence rates when compared to healthy population [10,11,12,13,14,15,16,17,18,19,20,21]. However, our results were contradictory to the results of the study by Lodyga et al., where they showed that patients of IBD had higher seroprevalence rates when compared to a healthy population [17]. Studies have shown variable effects of immunosuppressive drugs on seroprevalence rates of SARS-CoV-2 infection in patients of IBD. The CLARITY IBD cohort study showed patients receiving anti-TNFs such as infliximab had similar rates of infection, but low seroprevalence rates when compared to patients receiving vedolizumab, a gut selective anti-integrin and healthy population [14]. It indicates anti-TNFs impair the immune response to SARS-CoV-2 infection reducing the natural immunity and might predispose to reinfection. Hence, as per the CLARITY study, though the risk of infection was similar in patients with IBD regardless of the immunosuppressant use, the anti-TNF agents decreased the humoral immune response against the SARS-CoV-2 virus, which was responsible for lower seroprevalence in the anti-TNF-exposed patients. But none of the patients in our study was receiving anti-TNFs to study this effect. In the present study, the antibody titers were also comparable between patients with IBD and healthy controls and also between patients with or without immunosuppressant drug intake, indicating that neither the disease nor the immunosuppressant medications diminished the humoral immune response against SARS-CoV-2. Although the seroprevalence between patients with and without immunosuppressant use was similar in the entire IBD and UC cohort, patients with CD on immunosuppressants had a lower seroprevalence as compared to those without (20% vs. 50%, p = 0.01). This could be due to the small sample size, as only 46 and 22 patients, respectively, were with and without immunosuppressant at recruitment.
The strength of this study is its prospective nature, including a fair number of IBD patients from Asian population over a fairly long time period and having a comparator arm of healthy population. This data could avoid unnecessary fear among patients with IBD. Moreover, patients in this study were not vaccinated at the time of inclusion, which could avoid confounding with vaccination-induced seroconversion. Even though vaccination drive was started in India in January 2021, it was initially limited to healthcare workers, elderly and those with comorbidities until April 2021, the time-point at which our recruitment ended. Though we utilized anti-spike protein assay, the reported sensitivity and specificity of this assay was > 95% and in the absence of vaccination the interpretation of these results would not change [31]. However, in this study, the IBD group did not have patients receiving biologicals, which could not represent a uniform population of IBD. So, we could not study the effect of biologicals on seroprevalence, which is a limitation of this study. We also could not assess the serial immunological response in our patients, as the patients started getting vaccinated gradually. Though COVID-19 is regressing across the globe, the study provides a baseline seroprevalence of COVID-19 in patients with IBD from India and Indian seroprevalence was very different from global seroprevalence and that is a novel fact. India had one of the highest rates of infection across the globe and immunosuppression did not affect the risk of infection with SARS-Cov-2. Moreover, it is the first study of its kind from Asia.
To conclude, the seroprevalence rate of SARS-CoV-2 infection in patients of IBD was 32%. Disease and immunosuppressive drugs did not predispose to SARS-CoV-2 infection; neither did they affect the degree of humoral immunity. Preventive measures such as wearing a mask and maintaining social distancing are appropriate preventive measures for both patients with IBD and healthy people.
References
Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–9.
Singh P, Ananthakrishnan A, Ahuja V. Pivot to Asia: inflammatory bowel disease burden. Intest Res. 2017;15:138–41.
Kedia S, Ahuja V. Epidemiology of inflammatory bowel disease in India: the great shift east. Inflamm Intest Dis. 2017;2:102–15.
Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134–47.
Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021;15:879–913.
Rubin DT, Abreu MT, Rai V, et al. Management of patients with Crohn’s disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an international meeting. Gastroenterology. 2020;159:6-13.e6.
Seydoux E, Homad LJ, MacCamy AJ, et al. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. Immunity. 2020;53:98-105.e5.
Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–8.
Caini S, Bellerba F, Corso F, et al. Meta-analysis of diagnostic performance of serological tests for SARS-CoV-2 antibodies up to 25 April 2020 and public health implications. Euro Surveill. 2020;25:2000980.
Berte’ R, Mazza S, Stefanucci MR, et al. Seroprevalence of SARS-CoV2 in IBD patients treated with biologic therapy. J Crohns Colitis. 2021;15:864–8.
Freeman MC, Rapsinski GJ, Zilla ML, Wheeler SE. Immunocompromised seroprevalence and course of illness of SARS-CoV-2 in one pediatric quaternary care center. A J Pediatric Infect Dis Soc. 2021;10:426–31.
Simon D, Tascilar K, Krönke G, et al. Patients with immune-mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS-CoV-2 seroconversion. Nat Commun. 2020;11:3774.
Norsa L, Cosimo P, Indriolo A, Sansotta N, D’Antiga L, Callegaro A. Asymptomatic severe acute respiratory syndrome coronavirus 2 infection in patients with inflammatory bowel disease under biologic treatment. Gastroenterology. 2020;159:2229-31.e2.
Kennedy NA, Goodhand JR, Bewshea C, et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–75.
Scucchi L, Neri B, Sarmati L, et al. Low prevalence of SARS-CoV-2 infection in inflammatory bowel disease. Eur Rev Med Pharmacol Sci. 2021;25:2418–24.
McGregor CG, Adams A, Sadler R, et al. Maintenance therapy with infliximab or vedolizumab in IBD is not associated with increased SARS-CoV-2 seroprevalence: UK experience in the 2020 pandemic. Gut. 2021;70:2398–400.
Łodyga M, Maciejewska K, Eder P, et al. Inflammatory bowel disease is associated with higher seroprevalence rates of antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Pol Arch Intern Med. 2021;131:226–32.
Bossa F, Carparelli S, Latiano A, et al. Impact of the COVID-19 outbreak and the serum prevalence of SARS-CoV-2 antibodies in patients with inflammatory bowel disease treated with biologic drugs. Dig Liver Dis. 2021;53:277–82.
Dailey J, Kozhaya L, Dogan M, et al. Antibody responses to SARS-CoV-2 after infection or vaccination in children and young adults with inflammatory bowel disease. Inflamm Bowel Dis. 2022;28:1019–26.
Chanchlani N, Lin S, Chee D, et al. Adalimumab and infliximab impair SARS-CoV-2 antibody responses: results from a therapeutic drug monitoring study in 11 422 biologic-treated patients. J Crohns Colitis. 2022;16:389–97.
Di Ruscio M, Lunardi G, Buonfrate D, et al. A seroprevalence study of anti-SARS-CoV-2 antibodies in patients with inflammatory bowel disease during the second wave of the COVID-19 pandemic in Italy. Medicina (Kaunas). 2021;57:1048.
Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–64.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A.
Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32.
Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–44.
Murhekar MV, Bhatnagar T, Selvaraju S, et al. Prevalence of SARS-CoV-2 infection in India: findings from the national serosurvey, May-June 2020. Indian J Med Res. 2020;152:48–60.
Murhekar MV, Bhatnagar T, Selvaraju S, et al. SARS-CoV-2 antibody seroprevalence in India, August-September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health. 2021;9:e257–66.
Murhekar MV, Bhatnagar T, Thangaraj JWV, et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020-January 2021. Int J Infect Dis. 2021;108:145–55.
Murhekar MV, Bhatnagar T, Thangaraj JWV, et al. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June-July 2021: a population-based cross-sectional study. PLoS Med. 2021;18: e1003877.
Jahan N, Brahma A, Kumar MS, et al. Seroprevalence of IgG antibodies against SARS-CoV-2 in India, March 2020 to August 2021: a systematic review and meta-analysis. Int J Infect Dis. 2021;116:59–67.
National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020;20:1390–400.
Funding
AIIMS Intramural COVID Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BK, SKV, RG, TD, PK, SM, RG, SV, MV, GM, VA and SK declare no competing interests.
Ethics statement
The study was performed conforming to the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning human and animal rights and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kante, B., Vuyyuru, S.K., Gupta, R. et al. High seroprevalence against SARS-CoV-2 in non-vaccinated patients with inflammatory bowel disease from Northern India. Indian J Gastroenterol 42, 70–78 (2023). https://doi.org/10.1007/s12664-022-01310-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-022-01310-y