Abstract
Background and Aims
This meta-analysis of the randomized controlled trials was performed to assess effects of carnitine supplementation on serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels.
Methods
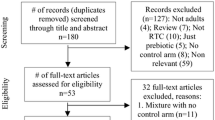
A comprehensive literature search of PubMed, Cochrane’s library, Web of Science, Scopus, and Embase was performed up to May 2018. From a total of 2012 articles identified initially, only 17 articles were included in the final meta-analysis to evaluate the effects of carnitine supplementation on serum levels of ALT and AST enzymes.
Results
Random effects model meta-analysis showed that carnitine supplementation led to reduction in serum ALT (weighted mean difference [WMD] − 10.25 IU/L; 95% CI = − 15.73, − 4.77; p < 0.001) and AST levels (WMD − 7.85 IU/L; 95% CI = − 11.85, − 3.86; p < 0.001). The results of subgroup analysis showed that carnitine could reduce serum AST levels at dosages equal to 2000 mg/day (p = 0.014) or more than 2000 mg/day (p < 0.001). However, carnitine supplementation at dosages of ≤ 1000 mg/day (p = 0.035) or equal to 2000 mg/day (p = 0.013) resulted in significant reduction in ALT level, while doses more than 2000 mg/day did not change ALT significantly. Carnitine exerts its reducing effect on serum ALT and AST levels only when these aminotransferases are raised or when the duration of supplementation lasts at least 3 months.
Conclusion
Results of the current meta-analysis showed that carnitine supplementation can decrease serum AST and ALT levels significantly, especially when supplementation lasts 3 months or more in patients with elevated serum aminotransferase levels.




Similar content being viewed by others
References
Rebouche CJ. Carnitine function and requirements during the life cycle. FASEB J. 1992;6:3379–86.
Alesci S, Manoli I, Costello R, et al. Carnitine: lessons from one hundred years of research. Ann N Y Acad Sci. 2004;1033.
Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond). 2010;7:30.
Gülcin İ. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78:803–11.
Boerrigter ME, Franceschi C, Arrigoni-Martelli E, Wei JY, Vijg J. The effect of L-carnitine and acetyl-L-carnitine on the disappearance of DNA single-strand breaks in human peripheral blood lymphocytes. Carcinogenesis. 1993;14:2131–6.
Gholipur-Shahraki T, Feizi A, Mortazavi M, Badri S. Effects of carnitine on nutritional parameters in patients with chronic kidney disease: an updated systematic review and meta-analysis. J Res Pharm Pract. 2018;7:57–68.
Veronese N, Stubbs B, Solmi M, Ajnakina O, Carvalho AF, Maggi S. Acetyl-L-carnitine supplementation and the treatment of depressive symptoms: a systematic review and meta-analysis. Psychosom Med. 2018;80:154–9.
Xu Y, Jiang W, Chen G, et al. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv Clin Exp Med. 2017;26:333–8.
Song X, Qu H, Yang Z, Rong J, Cai W, Zhou H. Efficacy and safety of L-carnitine treatment for chronic heart failure: a meta-analysis of randomized controlled trials. Biomed Res Int. 2017;2017:1–11.
Cave MC, Hurt RT, Frazier TH, et al. Obesity, inflammation, and the potential application of pharmaconutrition. Nutr Clin Pract. 2008;23:16–34.
Malaguarnera M, Vacante M, Giordano M, et al. L-carnitine supplementation improves hematological pattern in patients affected by HCV treated with Peg interferon-α 2b plus ribavirin. World J Gastroenterol 2011;17:4414–20.
Jun DW, Kim BI, Cho YK, et al. Efficacy and safety of entecavir plus carnitine complex (GODEX®) compared to entecavir monotherapy in patient with ALT elevated chronic hepatitis B: randomized, multicenter open-label trials. The GOAL study. Clin Mol Hepatol. 2013;19:165–72.
Hafkenscheid J, Dijt C. Determination of serum aminotransferases: activation by pyridoxal-5′-phosphate in relation to substrate concentration. Clin Chem. 1979;25:55–9.
Senior J. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury–past, present, and future. Clin Pharmacol Ther. 2012;92:332–9.
Alavinejad P, Zakerkish M, Hajiani E, Hashemi SJ, Chobineh M, Moghaddam EK. Evaluation of L-carnitine efficacy in the treatment of non-alcoholic fatty liver disease among diabetic patients: a randomized double blind pilot study. J Gastroenterol Hepatol Res. 2016;5:2191–5.
Malaguarnera M, Gargante MP, Cristaldi E, et al. Acetyl-L-carnitine treatment in minimal hepatic encephalopathy. Dig Dis Sci. 2008;53:3018–25.
Romano M, Vacante M, Cristaldi E, et al. L-carnitine treatment reduces steatosis in patients with chronic hepatitis C treated with α-interferon and ribavirin. Dig Dis Sci. 2008;53:1114–21.
Hassan A, Tsuda Y, Asai A, et al. Effects of oral L-carnitine on liver functions after transarterial chemoembolization in intermediate-stage HCC patients. Mediators Inflamm. 2015;2015:608216.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Delaš I, Dražić T, Čačić-Hribljan M, Sanković K. Effect of L-carnitine supplementation on some biochemical parameters in blood serum of sedentary population. Croat Chem Acta. 2008;81:163–8.
Georgala S, Schulpis KH, Georgala C, Michas T. L-carnitine supplementation in patients with cystic acne on isotretinoin therapy. J Eur Acad Dermatol Venereol. 1999;13:205–9.
Singh R, Niaz M, Agarwal P, Beegum R, Rastogi S, Sachan D. A randomised, double-blind, placebo-controlled trial of L-carnitine in suspected acute myocardial infarction. Postgrad Med J. 1996;72:45–50.
Somi MH, Fatahi E, Panahi J, Havasian MR. Data from a randomized and controlled trial of Lcarnitine prescription for the treatment for non-alcoholic fatty liver disease. Bioinformation. 2014;10:575–9.
Malaguarnera M, Vacante M, Giordano M, et al. Oral acetyl-l-carnitine therapy reduces fatigue in overt hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2011;93:799–808.
Malaguarnera M, Maugeri D, Saraceno B, et al. Effects of carnitine on biochemical responses in patients with chronic hepatitis C treated with interferon-α. Clin Drug Investig. 2002;22:443–8.
Malaguarnera M, Bella R, Vacante M, et al. Acetyl-L-carnitine reduces depression and improves quality of life in patients with minimal hepatic encephalopathy. Scand J Gastroenterol. 2011;46:750–9.
Malaguarnera M, Vacante M, Motta M, et al. Acetyl-L-carnitine improves cognitive functions in severe hepatic encephalopathy: a randomized and controlled clinical trial. Metab Brain Dis. 2011;26:281–9.
Malaguarnera M, Gargante MP, Russo C, et al. L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis—a randomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338–45.
Mosah HA, Khazaal FAK, Sahib HB, Hamdi AS. Effect of L-carnitine and raspberry ketones on metabolic parameters in Iraqi obese females, a comparative study. Int J Pharm Sci Rev Res. 2015;31:63–8.
Fukami K, Yamagishi S-I, Sakai K, et al. Potential inhibitory effects of L-carnitine supplementation on tissue advanced glycation end products in patients with hemodialysis. Rejuvenation Res. 2013;16:460–6.
Lim CY, Jun DW, Jang SS, Cho WK, Chae JD, Jun JH. Effects of carnitine on peripheral blood mitochondrial DNA copy number and liver function in non-alcoholic fatty liver disease. Korean J Gastroenterol. 2010;55:384–9.
An JH, Kim YJ, Kim KJ, et al. L-carnitine supplementation for the management of fatigue in patients with hypothyroidism on levothyroxine treatment: a randomized, double-blind, placebo-controlled trial. Endocr J. 2016;63:885–95.
Rebouche CJ. Kinetics, pharmacokinetics, and regulation of l-carnitine and acetyl-l-carnitine metabolism. Ann N Y Acad Sci. 2004;1033:30–41.
Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52.
Bowyer BA, Miles JM, Haymond MW, Fleming CR. L-carnitine therapy in home parenteral nutrition patients with abnormal liver tests and low plasma carnitine concentrations. Gastroenterology. 1988;94:434–8.
Alipour B, Barzegar A, Panahi F, Safaeian A., Es.haghi M. Effect of L-carnitine supplementation on metabolic status in obese diabetic women with hypocaloric diet. Health Scope. 2014;3:e14615
González-Ortiz M, Hernández-González SO, Hernández-Salazar E, Martínez-Abundis E. Effect of oral L-carnitine administration on insulin sensitivity and lipid profile in type 2 diabetes mellitus patients. Ann Nutr Metab. 2008;52:335–8.
Kraemer WJ, Volek JS, French DN, et al. The effects of L-carnitine L-tartrate supplementation on hormonal responses to resistance exercise and recovery. J Strength Cond Res. 2003;17:455–62.
Söderberg C, Stål P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602.
Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–86.
Moghadam SA, Nematy M, Eghtesadi S, et al. Effects of L-carnitine supplementation on inflammatory factors and malondialdehyde in patients with nonalcoholic steatohepatitis (NASH). Curr Top Nutraceut Res. 2015;13:135–42.
Albano E, Mottaran E, Vidali M et al. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987–93.
Acknowledgements
The proposal of this systematic review and meta-analysis was approved in Student Research Committee of Lorestan University of Medical Sciences, Khorramabad, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
EYR, EE, EF, MM, AH, OA, and SS declare that they have no conflict of interest.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yousefi Rad, E., Eslampour, E., Falahi, E. et al. Effects of carnitine supplementation on liver aminotransferase enzymes: A systematic review and meta-analysis of randomized controlled clinical trials. Indian J Gastroenterol 38, 470–479 (2019). https://doi.org/10.1007/s12664-019-00983-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-019-00983-2