Abstract
Purpose
Amylases are environmentally attractive enzymes and are employed in food processing technology; for example, the starch hydrolysis process is used for glucose syrup production. The use of biocatalysts attached to a support promotes greater stabilization of the enzyme and allows its reuse, this being interesting for industrial applications. The choice of the support for enzyme immobilization must be made according to the process costs, therefore, the employment of agro-industrial byproducts is an alternative. We evaluated the immobilization of commercial glucoamylase in corncob powder (CCP) and the application of the immobilized enzyme (CCP-glucoamylase) in response to starch hydrolysis process
Results
The yield of glucoamylase immobilization in CCP was 95%. Soluble glucoamylase and CCP-glucoamylase presented maximum activity temperatures at 50 °C and 60 °C, respectively. Both biocatalysts showed a maximum activity at pH 5. Soluble glucoamylase and CCP-glucoamylase exhibited a good thermal stability at 50 °C, resulting in half-lives of 33 h and 46 h, respectively. Soluble glucoamylase and CCP-glucoamylase reached starch conversion into glucose of 75% and 16%, respectively, after 12 h of reaction, with an enzymatic load of 30 U per gram of starch. However, after an increase in the enzymatic load of CCP-glucoamylase, starch conversion was equivalent
Conclusion
CCP-glucoamylase has the potential to hydrolyze starch into glucose and can be reused, thus becoming an enhanced value-added bioproduct and an alternative for industrial applications. A strategy for future industrial application would be the hydrolysis of starch using CCP-glucoamylase in a fixed bed reactor operated continuously.
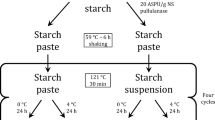
Graphic Abstract








Similar content being viewed by others
Data Availability
We are providing a document with supplementary information.
Abbreviations
- CCP:
-
Corn cob powder
- CCP-glutaraldehyde:
-
Activated support
- CCP-glucoamylase:
-
Immobilized glucoamylase
- ATR-FTIR:
-
Attenuated Total Reflection-Fourier Transform Infrared
- U:
-
International units
- HPLC:
-
High-performance liquid chromatography system
- DNS:
-
3,5-Dinitrosalicylic acid reagent
- MOS:
-
Malto-oligossaccharides
- M1 :
-
Glucose
- M2 :
-
Maltose
- M3 :
-
Maltotriose
- M4 :
-
Maltotetraose
- M5 :
-
Maltopentaose
- M6 :
-
Maltohexaose
- M7 :
-
Maltoheptaose
- M8 :
-
Maltooctaose
- Kd:
-
Enzyme deactivation
- t½:
-
Half-life
- Rp:
-
Yield by protein quantification
- RA :
-
Yield per enzyme activity
- E:
-
Immobilization efficiency
- AR :
-
Recovered activity
- Ae:
-
Enzymatic activity of immobilized glucoamylase
- EDA:
-
Ethylenediamine
- BSA:
-
Bovine serum albumin
- SDS-PAGE:
-
Polyacrylamide gel electrophoresis with sodium dodecyl sulfate
References
Abraham, M.: Encycloppedia of sustainable technologies. Elsevier, Amsterdam (2017)
Sumerly, R., Alvarez, H., Cereda, M.P., Vilpoux, O.: Tecnologias, usos e potencialidades de tuberosas amiláceas latino americanas. Fundação Cargill, São Paulo (2003)
Pasin, T.M., Benassi, V.M., Heinen, P.R., de Damasio, A.R., L., Cereia, M., Jorge, J.A., Polizeli, M. de L.T. de M. : Purification and functional properties of a novel glucoamylase activated by manganese and lead produced by Aspergillus japonicus. Int. J. Biol. Macromol. 102, 779–788 (2017). https://doi.org/10.1016/j.ijbiomac.2017.04.016
Guzmán-Maldonado, H., Paredes-López, O.: Amylolytic enzymes and products derived from starch: a review. Crit. Rev. Food Sci. Nutr. 35, 373–403 (1995). https://doi.org/10.1080/10408399509527706
Kumar Singh, S., Khan, A., Nath Singh, R., Bahuguna, A., Chauhan, P., Kumar Sharma, V., Kaur, S.: Production, purification and characterization of thermostable α-amylase from soil isolate Bacillus sp. strain B-10. J. BioSci. Biotechnol. 5, 37–43 (2016). https://doi.org/10.1208/s12249-011-9586-1
Pandey, A., Nigam, P., Soccol, C.R., Soccol, V.T., Singh, D., Mohan, R.: Advances in microbial amylases. Biotechnol. Appl. Biochem. 31, 135–152 (2000). https://doi.org/10.1042/ba19990073
Almeida, P.Z., Messias, J.M., Pereira, M.G., Pinheiro, V.E., Maria, L., Monteiro, O., Ricardo, P., Cardoso, G.C., Jorge, J.A., Lourdes, M.D., Zaghetto, P., Messias, J.M., Pereira, M.G., Pinheiro, V.E., Maria, L., Monteiro, O., Heinen, P.R., Cunha, G.: Mixture design of starchy substrates hydrolysis by an immobilized glucoamylase from Aspergillus brasiliensis. Biocatal. Biotransformation. 36, 389–395 (2018). https://doi.org/10.1080/10242422.2017.1423059
van der Maarel, M.J.E.C., van der Veen, B., Uitdehaag, J.C.M., Leemhuis, H., Dijkhuizen, L.: Properties and applications of starch-converting enzymes of the alpha-amylase family. J. Biotechnol. 94, 137–155 (2002). https://doi.org/10.1016/s0168-1656(01)00407-2
Roy, I., Gupta, M.N.: Hydrolysis of starch by a mixture of glucoamylase and pullulanase entrapped individually in calcium alginate beads. Enzyme Microb. Technol. 34, 26–32 (2004). https://doi.org/10.1016/j.enzmictec.2003.07.001
Chapman, J., Ismail, A.E., Dinu, C.Z.: Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts. 8, 20–29 (2018). https://doi.org/10.3390/catal8060238
Hassan, M.E., Yang, Q., Xiao, Z., Liu, L., Wang, N., Cui, X., Yang, L.: Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech. 9, 1–16 (2019). https://doi.org/10.1007/s13205-019-1969-0
Katchalski-Katzir, E.: Immobilized enzymes-learning from past successes and failures. Trends Biotechnol. 11, 471–478 (1993). https://doi.org/10.1016/0167-7799(93)90080-S
Cao, L.: Immobilised enzymes: science or art? Curr. Opin. Chem. Biol. (2005). https://doi.org/10.1016/j.cbpa.2005.02.014
Hassan, M.E., Yang, Q., Xiao, Z.: Covalent immobilization of glucoamylase enzyme onto chemically activated surface of κ-carrageenan. Bull. Natl. Res. Cent. 43, 2–11 (2019). https://doi.org/10.1186/s42269-019-0148-0
de Aguiar, R.O., Mondardo, R.M., Agnes, E.J., de Castro, H.F., Pereira, E.B.: Avaliação e comparação da eficiência de imobilização de lipase pancreática em quitosana para produção de ácidos graxos em frascos agitados. Acta Sci. Technol. 32, 15–19 (2010). https://doi.org/10.4025/actascitechnol.v32i1.7546
Bassan, J., de Souza Bezerra, T., Peixoto, G., da Cruz, C., Galán, J., Vaz, A., Garrido, S., Filice, M., Monti, R.: Immobilization of Trypsin in Lignocellulosic Waste Material to Produce Peptides with Bioactive Potential from Whey Protein. Materials (Basel). 9, 357 (2016). https://doi.org/10.3390/ma9050357
Dalla-Vecchia, R., Nascimento, M.D.G., Soldi, V.: Aplicações sintéticas de lipases imobilizadas em polímeros. Química Nova (2004). https://doi.org/10.1590/S0100-40422004000400017
Bilal, M., Iqbal, H.M.N.: Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities – A review. Food Res. Int. 123, 226–240 (2019). https://doi.org/10.1016/j.foodres.2019.04.066
Bradford, M.M.: A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 254, 248–254 (1976). https://doi.org/10.1016/0003-2697(76)90527-3
de Almeida, P.Z., Pereira, M.G., de Carvalho, C.C., Heinen, P.R., Ziotti, L.S., Messias, J.M., Jorge, J.A., de Polizeli, M.: Bioprospection and characterization of the amylolytic activity by filamentous fungi from Brazilian Atlantic Forest. Biota Neotrop. 17, 2–9 (2017). https://doi.org/10.1590/1676-0611-bn-2017-0337
Tavano, O.L., Pessela, B.C.C., Goulart, A.J., Fernández-Lafuente, R., Guisán, J.M., Monti, R.: Stabilization of an amylase from Neurospora crassa by Immobilization on Highly Activated Supports. Food Biotechnol. 22, 262–275 (2008). https://doi.org/10.1080/08905430802262616
Miller, G.L.: Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 31, 426–428 (1959). https://doi.org/10.1021/ac60147a030
Sheldon, R.A., van Pelt, S.: Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev. 42, 6223–6235 (2013). https://doi.org/10.1039/c3cs60075k
Laemmli, U.K.: Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227, 680–685 (1970). https://doi.org/10.1038/227680a0
McIlvaine, T.C.: A BUFFER SOLUTION FOR COLORIMETRIC COMPARISON. J. Biol. Chem. (1921). https://doi.org/10.1016/S0021-9258(18)86000-8
Oliveira, S.C., Paz-Cedeno, F.R., Masarin, F. (2017): Kinetic modeling of monomeric sugars formation during the enzymatic hydrolysis of the residue generated in the carrageenan production from algal biomass. In: XXI SINAFERM. , Aracaju
Oliveira, S.C., Paz-Cedeno, F.R., Masarin, F.: Mathematical modeling of glucose accumulation during enzymatic hydrolysis of carrageenan waste. In: Silva Santos, A. (ed.) Avanços científicos e tecnológicos em bioprocessos, pp. 97–103. Brazil, Atena Editora (2018)
Solorzano-Chavez, E.G., Paz-Cedeno, F.R., Ezequiel de Oliveira, L., Gelli, V.C., Monti, R., Conceição de Oliveira, S., Masarin, F.: Evaluation of the Kappaphycus alvarezii growth under different environmental conditions and efficiency of the enzymatic hydrolysis of the residue generated in the carrageenan processing. Biomass Bioenerg. (2019). https://doi.org/10.1016/j.biombioe.2019.105254
Paz-Cedeno, F.R., Solórzano-Chávez, E.G., de Oliveira, L.E., Gelli, V.C., Monti, R., de Oliveira, S.C., Masarin, F.: Sequential Enzymatic and Mild-Acid Hydrolysis of By-Product of Carrageenan Process from Kappaphycus alvarezii. Bioenergy Res. (2019). https://doi.org/10.1007/s12155-019-09968-7
Melo, E.P.: Estabilidade de proteínas. In: Cabral, J.M.S., Aires-barros, M.R., Gama, M. (eds.) Engenharia enzimática, pp. 67–120. Lidel, Lisbon (2003)
Xian, L., Feng, J.X.: Purification and biochemical characterization of a novel mesophilic glucoamylase from Aspergillus tritici WZ99. Int J Biol Macromol. 107, 1122–1130 (2018). https://doi.org/10.1016/j.ijbiomac.2017.09.095
Pavezzi, F.C.: Produção e caracterização de glucoamilases termoestáveis de Aspergillus awamori obtidas por PCR mutagênico e expressas em Saccharomyces cerevisiae. Universidade Estadual Paulista, Sao Paulo (2006)
Riaz, M., Rashid, M.H., Sawyer, L., Akhtar, S., Javed, M.R., Nadeem, H., Wear, M.: Physiochemical properties and kinetics of glucoamylase produced from deoxy-d-glucose resistant mutant of Aspergillus niger for soluble starch hydrolysis. Food Chem. 130, 24–30 (2012). https://doi.org/10.1016/j.foodchem.2011.06.037
Parashar, D., Satyanarayana, T.: Engineering a chimeric acid-stable α-amylase-glucoamylase (Amy-Glu) for one step starch saccharification. Int. J. Biol. Macromol. 99, 274–281 (2017). https://doi.org/10.1016/j.ijbiomac.2017.02.083
Aalbæk, T., Reeslev, M., Jensen∗, B., Eriksen, S.H. : Acid protease and formation of multiple forms of glucoamylase in batch and continuous cultures of Aspergillus niger. Enzyme Microb. Technol. 30, 410–415 (2002). https://doi.org/10.1016/S0141-0229(02)00006-6
Santos, A. (2010): Imobilização de invertase comercial e de Saccharomyces cerevisiae em sabugo de milho e bagaço de cana-de-açúcar,
Sheldon, R.A., van Pelt, S.: Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 42, 6223–6235 (2013). https://doi.org/10.1039/c3cs60075k
Boudrant, J., Woodley, J.M., Fernandez-Lafuente, R.: Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 90, 66–80 (2020). https://doi.org/10.1016/j.procbio.2019.11.026
Shetty, P., Jaffer, M.: Chemical coupling of glucoamylase produced by Arthrobotrys conoides onto cotton cloth and Ocimum basilicum seeds and characterization of the immobilized enzyme. J. Microbiol. Biotechnol. Food Sci. 6, 1127–1131 (2017). https://doi.org/10.15414/jmbfs.2017.6.5.1127-1131
Roldán, I.U.M., Mitsuhara, A.T., Munhoz Desajacomo, J.P., de Oliveira, L.E., Gelli, V.C., Monti, R., Silva do Sacramento, L.V., Masarin, F. : Chemical, structural, and ultrastructural analysis of waste from the carrageenan and sugar-bioethanol processes for future bioenergy generation. Biomass Bioenerg. 107, 233–243 (2017). https://doi.org/10.1016/j.biombioe.2017.10.008
Silverstain, R., Webster, F., Kiemle, D.: Identificação Espectrométrica de Compostos Orgánicos. LTC, Rio de Janeiro (2006)
Bagheri, A., Khodarahmi, R., Mostafaie, A.: Purification and biochemical characterisation of glucoamylase from a newly isolated Aspergillus niger: Relation to starch processing. Food Chem. 161, 270–278 (2014). https://doi.org/10.1016/j.foodchem.2014.03.095
Syed, F., Ali, K., Javaid, M., Gul, M., Khan, Z., Imran, M., Taj, R., Ahmad, A.: Journal of Photochemistry & Photobiology, B : Biology Preparation and characterization of a green nano-support for the covalent immobilization of glucoamylase from Neurospora sitophila. JPB. 162, 309–317 (2016). https://doi.org/10.1016/j.jphotobiol.2016.07.002
Cardoso, C.L., de Moraes e Queiza, M.C.: Imobilização de enzimas em suportes cromatográficos: uma ferramenta na busca por substâncias bioativas. Quim. Nova. 32, 175–187 (2009)
Li, X.D., Wu, J., Jia, D.C., Wan, Y.H., Yang, N., Qiao, M.: Preparation of cross-linked glucoamylase aggregates immobilization by using dextrin and xanthan gum as protecting agents. Catalysts. (2016). https://doi.org/10.3390/catal6060077
George, R., Gopinath, S., Sugunan, S.: Improved Stabilities of Immobilized Glucoamylase on Functionalized Mesoporous Silica Synthesised using Decane as Swelling Agent. Bull. Chem. React. Eng. & Catal. (2013). https://doi.org/10.9767/bcrec.8.1.4208.70-76.(2013)
Nadar, S.S., Rathod, V.K.: Facile synthesis of glucoamylase embedded metal-organic frameworks (glucoamylase-MOF) with enhanced stability. Int. J. Biol. Macromol. 95, 511–519 (2017). https://doi.org/10.1016/j.ijbiomac.2016.11.084
Costa, C.Z., Albuquerque, M.C.C., Brum, M.C., Castro, A.M.: Degradação microbiológica e enzimática de polímeros: uma revisão. Química Nova (2015). https://doi.org/10.5935/0100-4042.20140293
Zhong, N., Chen, W., Liu, L., Chen, H.: Immobilization of Rhizomucor miehei lipase onto the organic functionalized SBA-15: Their enzymatic properties and glycerolysis efficiencies for diacylglycerols production. Food Chem. 271, 739–746 (2019)
Agustian, J., Hermide, L.: Saccharification kinetics at optimised conditions of tapioca by glucoamylase immobilized on mesostructure cellular foam sílica. Eurasian Chem. J. (2018). https://doi.org/10.18321/ectj764
Acknowledgements
FAPESP (contract number 2018/06241-3) funding this work. Coordination of Improvement of Higher Education Personnel (CAPES) funding the doctoral scholarship of Fernando Roberto Paz-Cedeno and master scholarship of Isabela Costa Luchiari.
Funding
São Paulo State Research Support Foundation (FAPESP, contract number 2018/06241–3), funding this work. Coordination of Improvement of Higher Education Personnel (CAPES) funding the master Isabela da Costa Luchiari and doctoral scholarship of Fernando Roberto Paz-Cedeno.
Author information
Authors and Affiliations
Contributions
ICL, FRPC, TAMF and FPP immobilization and characterization of glucoamylase, evaluation of thermal and pH stability, enzymatic hydrolysis of starch, analyses of samples. ICL, FRPC, TAMF, FPP, AVP, RM and FM participated in the reviewed the manuscript and data interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Costa Luchiari, I., Cedeno, F.R.P., de Macêdo Farias, T.A. et al. Glucoamylase Immobilization in Corncob Powder: Assessment of Enzymatic Hydrolysis of Starch in the Production of Glucose. Waste Biomass Valor 12, 5491–5504 (2021). https://doi.org/10.1007/s12649-021-01379-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01379-0