Abstract
Atmospheric microwave plasma was applied to the processing of the partially cleaned producer gas obtained from sewage sludge gasification. The plasma processing resulted in residual tar compounds conversion and changes in the gas composition. During the tests with a different gas flow rates and microwave power inputs, liquid and gaseous samples were collected to evaluate the plasma reactor’s performance. The conversion efficiency ranged from 19 to 100% and it depended on the specific energy input (SEI), gas flow rate, initial tar concentration, and the nature of the tars compounds. Generally, it was shown that the conversion rate increased with the SEI and that the aliphatic, cyclic and substituted compounds were converted much easier than benzene. Moreover, applying plasma led to the production of heavier aromatics (i.e. naphthalene, indene, acenaphthylene) but the rise in their concentration was significantly smaller than the amount of converted compounds. The gas composition changes revealed in the increase of H2 and CO concentration that was an effect of hydrocarbons and CO2 conversion. Additionally, it was indicated that the microwave plasma reactor’s performance was noticeably worse than in the case of the laboratory test with a simulated producer gas. This was mainly attributed to differences in the reactors’ geometry, lower hydrogen concentration and the presence of inorganic deposit on the reactor’s walls that might have inhibited microwaves transfer. In general, the microwave plasma technology seems promising in the context of cleaning and upgrading the producer gas, however, further optimization research is necessary to make it more reliable and less energy consuming.
Graphic Abstract

Similar content being viewed by others
Statement of Novelty
Biomass-derived producer gas treatment in the context of tar conversion is a current and important research issue. Many plasma techniques have been investigated to solve the problem of tar’s presence. However, most of them considered simplified model mixtures of gas and a model tar compound. There is a negligible amount of research that applies a real producer gas obtained in the gasification process. Moreover, none of them includes microwave plasma, which has a few promising features considering producer gas valorization. Therefore, the main goal and novelty of this work were to investigate the microwave plasma treatment of the gas derived from sewage sludge gasification and to compare it with the results obtain in laboratory research using model mixtures.
Introduction
Biomass gasification is gaining rapt attention in the context of renewable energy and sustainable development [1, 2]. One of the reasons for it is that gasification can be considered as one of the most flexible fuel conversion processes. The producer gas can be utilized in boilers, engines, turbines, or even fuel cells [3]. Moreover, it can be converted into syngas or hydrogen which might be applied in chemical syntheses of many products, including components of liquid fuels, methanol, and fertilizers [4]. Additionally, biomass can be considered as a renewable source (neutral in terms of CO2 emission [2, 5]) that is widely and relatively evenly distributed along the earth thus accessible locally [5]. The biomass gasification may become even more attractive if wastes were considered as the gasification fuel. Gasification of the sewage sludge could be a good example of such an approach.
The abundancy of sewage sludge is now a major concern in many countries [6,7,8]. The development of sewage treatment plants, being a result of directives and large financial expenditures of the European Union [8, 9], results in a significant increase in the production of sewage sludge [7, 8, 10]. Moreover, there are directives that significantly limit or even completely prohibit landfilling of the sewage sludge [7, 11]. The use of sewage sludge as fertilizers is also significantly limited [7, 9], due to a high content of heavy metals and pathogens contained in it [6, 7]. A solution to this was found in thermal processing of sewage sludge. The most common approach is the incineration [7, 12], but it involves the emission of significant amounts of pollutants such as SO2, NOx, and heavy metals [7, 13]. Alternatively, sewage sludge could be processed via gasification resulting in the production of valuable producer gas of flexible utility [7, 10, 11].
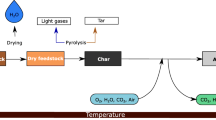
Despite its advantages, biomass gasification is inseparably connected with tars production and sewage sludge gasification is no exception. Tars may be defined in many ways and assigned to a few categories but generally, they are a mixture of heavy organic compounds—mainly aromatics [14, 15]. These compounds tend to condensate at the temperature range of 150–350 °C [15, 16] and elevated pressure [17] thus resulting in malfunctions of mechanical devices as well as fouling and blocking of pipelines and filters [15, 18]. In fact, tars may be considered as a waste stream from the gasification process, that is the main obstacle in the process commercialization [14, 18, 19]. The most common approach to remove tars from the gas stream is to use mechanical, usually wet methods that involve scrubbers or washing towers [15, 19, 20]. However, this method does not solve the tars problem completely but instead “pushes” it away creating a wastewater stream that consists of tars [19]. A more proper approach involves tar conversion rather than its removal. Since the yield of tars may vary from few to even 10 wt% (or more) [14] they may carry a considerable amount of energy. Methods that can allow for tar conversion may be classified into thermal, catalytic, and plasma methods [18]. While the thermal methods seem to be the most simple, they require a very high temperature (e.g. 1200 °C [21, 22]) that may be hard to achieve and can cause material problems. Catalytic methods require a significantly lower temperature of ca. 500–700 °C [15, 23] but the catalyst itself may be expensive and vulnerable to poisoning and deactivation (as it is in the case of the most commonly tested nickel catalyst [23, 24]). The poisoning may be especially problematic in the case of sewage sludge gasification, due to its high content of S and N [12, 13]. Sulfur and nitrogen compounds present in the produced gas are a well-known catalyst poison [25,26,27]. Plasma methods, although the most expensive in terms of investment and operational cost, do not suffer from the drawback that can be attributed to other conversion methods. In fact, they might be considered as a specific hybrid of catalytic and thermal methods due to a usually high temperature of plasma and the presence of reactive species (e.g. radicals, electrons, ions, and excited molecules), that may significantly enhance the decomposition process [18, 28]. Consequently, similarly to catalytic methods, plasma may provide high conversion rate of tars into valuable products that will result in a general improvement of biomass conversion. However, the main drawbacks of plasma methods are high investment and operational costs. The operational costs are mostly connected with electric energy consumption thus a significant cost reduction could have been achieved if the plasma methods would be coupled with renewable energy sources. Such a connection seems especially appropriate since the quick start/stop procedure of plasma reactors is advantageous in terms of quickly changing natural sources of energy (wind of sunlight) [29]. Energy efficiency of plasma reactors variates widely depending on the process conditions and plasma types, but the recent research show that as high values as 20–60 g of converted tar compounds per kWh could be achieved [29, 30] with the specific energy input (SEI—ratio between power supply and volumetric gas flow rate, see “Assessment Methods”) section usually below 1 kWh/Nm3. With the SEI close to 1 kWh/Nm3 or even higher, it can be concluded that application of plasma method is not reasonable in case of air gasification with the gas calorific values usually around 4–6 MJ/Nm3 (1.11–1.67 kWh/Nm3) [14]. However, the application of high SEI plasma could be justified in the context of more valuable products, like syngas, hydrogen and subsequent chemicals. Many plasma techniques have been investigated in terms of tars conversion, e.g. corona discharge [31, 32], dielectric barrier discharge [33, 34], arc plasma [12, 35], gliding arc plasma [36,37,38], and microwave plasma (MWP) [28, 39,40,41]. The previous research of the authors proved that MWP might allow high conversion of tar compounds, while at the same time it influences gas composition increasing the content of H2 and CO at the cost of CO2 and hydrocarbons [28, 40]. This can be achieved not only by a high temperature of the atmospheric MWP, which can usually range from ca. 5000 to 6000 K, but also due to the high concentration of radicals, i.e. O, OH, and H, that enhanced the conversion reaction [28, 40, 42]. MWP may also be considered as a promising method for tar conversion due to its electrodeless character (electrodes are the most life-limiting factor in plasma techniques [18], what may be especially problematic in the context of aggressive producer gas conditions), relatively cheap components [43], and the possibility for scaling up due to commercial production of microwave generators with a wide range of power from few to hundreds of kW.
Although there is a lot of research on plasma application in tar conversion, most of it involves simplified conditions, e.g. using nitrogen and/or argon as a plasma gas or model tar compounds like toluene. There are very few articles where a real producer gas [12, 31, 35] is applied and none of them involve MWP. While the authors’ recently published article [28] presents the results from the lab-scale MWP tar conversion in the atmosphere of simulated producer gas, it is still quite a simplification. Therefore, the goal of the experiments presented in this article was to test the MWP tar conversion when a real producer gas, derived from sewage sludge gasification, was applied. The motivation behind those works was to verify the results from simplified, simulate, lab-scale experiments [28, 40] and to recognize any potential problems that may arise when a real producer gas is converted in the MWP reactor.
Experimental Section
Experimental Setup
For the purpose of the experiment, REMIX S.A. company (Poland, Świebodzin) provided a test facility with a gasifier. The pilot-scale gasification plant investigated in this research was composed of four basic units: supply unit of feedstock, gasifier itself, producer gas cleaning line, and the furnace for the utilization of unburnt pellets. The gasified material was prepared from dried sewage sludge in the form of pellets with the diameter of 6 and 8 mm and stored in a silo, from which they were conveyed to the fuel hopper on top of the gasifier. The gasification reactor was the downdraft gasifier made of heat-resistant steel with an internal diameter of 0.42 m and a height of 1.82 m. The pilot plant operated periodically: the prepared batch of sewage sludge pellets was loaded into a sealed container from which the feedstock was delivered to the gasifier by means of a cellular feeder and bucket elevator with a capacity of 20 kg/h. Air electrically heated up to 400 °C was supplied by the compressor to the reactor throat as a gasifying agent with a capacity of about 20 Nm3/h. The average equivalence ratio (ER—ratio of air used to stoichiometric air) was 0.4. The incompletely gasified char-pellets were burned-out in the BFB furnace. The amount of char collected after gasification was approximately 60% in relation to the mass of pellets.
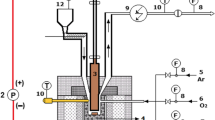
Figure 1 presents the scheme of the producer gas cleaning line. In the initial step, the gas was cleaned with the use of mechanical methods that include cyclone, disintegrator (centrifuge), two scrubbers (one with oil and one with water), demister, and a filter with sawdust. After passing the initial cleaning step, the gas was heated up to 120 °C to prevent condensation of water at the inlet of the MWP reactor.
The principle of work of all atmospheric, electrodeless MWP reactors is identical [28, 39, 41, 42]. The plasma discharge is initiated and sustained due to absorption of microwave radiation. The microwave generator provides radiation (in this case with the frequency of 2.45 GHz) that transfers through a waveguide. This kind of plasma reactors may work without electrodes and the plasma is generated in a quartz tube where microwave radiation affects flowing gas. The only exception to the electrodeless character of the reactor is the moment of plasma ignition. During this short stage, a tungsten rod is introduced into the quartz tube to focus microwave energy and provide ionization of the gas and further development of the plasma discharge. After the ignition, the rod is removed from the reactor. The plasma gas is introduced into the reactor tangentially, what increases the stability of the plasma discharge and protects the reactor’s walls from contact with the plasma. Practically, most of the electrodeless, atmospheric plasma reactors have very similar construction [28, 39, 41, 42] but some differences may occur in the geometry of the reactor, the generator’s power and the presence of additional auxiliary devices. The major difference in the case of presented results was using four MW generators in series. The upper two generators were of 5 kW power while the two lower ones were of 3 kW. Since the microwave generation efficiency is ca. 60%, the microwave power was 3 kW and 2 kW, respectively. Each of the microwave generation lines ended in a movable plunger which allows optimizing microwave absorption/reflection ratio [42]. The reactor’s quartz tube was 1500 mm length and its inner diameter was 36 mm. The quartz tube was cooled with the use of five auxiliary fans located alongside the reactor. The purpose of cooling was to prevent overheating of the quartz and damaging of the reactor. For the same reason, no thermal insulation was applied. The MWP reactor was designed and manufactured by PROMIS-TECH Sp. z o.o. (Wrocław, Poland).
At the outlet of the reactor, a two-stage cooler (fed with air and water) was connected. After the cooler, the gas was passing a gas meter (Intergaz, BK-G16 M) and eventually was stored in gasbag or burned in a flare.
Material
The feedstock, sewage sludge, was obtained from a wastewater treatment facility in Świebodzin (Poland). The material was dried and pelletized at REMIX S.A. test facility prior to gasification. The properties of the sewage sludge are presented in Table 1.
Experimental Procedure
The initial operating parameters of the gasifier were as mentioned above (“Experimental Setup” section). After initiation of the gasification process with the use of hot air (400 °C), the air feed was controlled to keep the reduction zone temperature at the level of 725 ± 50 °C. After reaching a reasonable repeatability of the temperature and the gas composition (usually 3–4 h after the start-up), the producer gas (preliminarily cleaned by with mechanical methods) was passed through the MWP reactor as the only plasma gas. Prior to that, the MWP reactor was started-up and worked for ca. 10 min on air, that was gradually shut down with the producer gas introduction.
The whole experiment concerning MWP reactor application in gas cleaning was divided into two stages. During the first stage, only two upper microwave generators were applied resulting in microwave power of 3 and 6 kW, respectively. The second stage was performed with the use of three and all four generators thus the microwave power was respectively 8 and 10 kW. These two stages have been distinguished due to the experiment’s schedule and some problems considering matching all four generators and providing plasma discharge development along them. The MWP reactor operation time was ca. 40 min in case of each stage. It should be also noted that the main parameter used to control the gasification process was the temperature inside the bed. This temperature was controlled by the stream of gasifying agent—air. As a result, the gas flow rate in the MWP reactor changed as well. Additionally, partial blocking of the producer gas cleaning line filters and pellets sintering in the reactor also could have affected the gas flow rate. Table 2 summarizes the conditions of all four experiments with different microwave power, gas flow rate, and resulting SEI. Since the gasification was performed in a periodic, semi-continuous fix bed gasifier, the gas composition could have varied within the process. Moreover, as it can be seen in Table 2 even the gas flow rate could vary significantly depending on the run. Due to hard to achieve stability and repeatability of the process, as well as the time-consuming character of the experiments, the sampling of the gas was done only once for every case. As a result, no error analyses were performed and it should be noted that the results presented in the further part of the article might be burdened with a high uncertainty. Nevertheless, the results show clear trends and they might be used for comparison with previous results or provide valuable leads considering process improvement.
For the purpose of composition analyses, the gas was sampled before MWP reactor (1st sampling point in Fig. 1) and after passing it (2nd sampling point in Fig. 1). In the latter case, the sampling was done twice for each SEI applied in the stage. The gas was sampled with the use of a conditioning unit (M&C, PSS 5/3) equipped with a peristaltic pump (180 NL/min) for 10 min. Before reaching the conditioner, the gas was passing through an absorption unit that consisted of three Dreschle flasks filled with isopropanol (75 mL) and kept in a chiller (− 10 °C) (PolyScience, SD07R-20). Additionally, the conditioner was followed by a gas analyzer (GEIT, GAS 3100R) allowing for the measurement of CO, CO2, and H2. Finally, the gas was pumped into the Tedlar bag (5 L) and transported to a laboratory for the gas chromatograph (GC) analyses. The GC analyses were applied for both gas and liquid samples. The liquid samples (a mixture of isopropanol with tars) were analyzed with the use of GC (Agilent 7820) equipped with a mass spectrometer (Agilent MSD 5977) and HP-5M column. These analyses allowed identifying the tar compounds in the producer gas and quantifying some of them. The GC (HP 6980) equipped with a flame ionization detector (FID) and RT-Alumnia Bond KCl column (Restek) was used for gas analyses. The purpose of these analyses was to identify and quantify the main light hydrocarbons (C1–C3) present in the producer gas. More information considering chromatographic analyses, including temperature programs, can be found in the previous work [40]. All the GC samples were analyzed at least three times. The results of the GC analyzes present average values with standard deviation.
Measurements of particles concentration in the producer gas were made using the gravimetric method according to the Polish standard (PN-Z-04030-7). This method base on an isokinetic suction of the gas through a fiberglass filter (in 1st sampling point in Fig. 1). The sampling was done with the use of gravimetric dust meter (EMIO, EMIOTEST 2598). Samples of collected dust were dried to evaporate water at 105 °C and then at the temperature of 200 °C to remove organic compounds. Knowing the total gas flow rate, the final mass of the filter was used to calculate the particles concentration. The dust sampling was done twice.
Additionally, a residue deposited on the walls of the reactor during the plasma processing was collected and analyzed with the use of scanning electron microscope (SEM) (Phenom, XL) equipped with energy dispersive spectroscopy (EDS) detector and X-ray diffraction method (XRD) with the use of symmetric θ/2θ Bragg–Brentano geometry system (Philips, X’PERT).
The analyses of the dried sewage sludge (see Table 1) and its ash (see Table 7) were done by an external laboratory (Laboratory of Fuels and Activated Carbons, Institute for Chemical Processing of Coal, Zabrze, Poland).
Assessment Methods
The conversion efficiency (η) of tars compounds was calculated as follows:
where, C0—refers to the initial, inlet concentration of converted compound (i.e. benzene, toluene) (g/Nm3), C—refers to final, outlet concentration of converted compounds after MWP processing (g/Nm3).
Alternatively, a simplified approach for estimation of the conversion efficiency may have involved area of the peaks from GC analyses instead of the actual concentration. In that case, concentrations (C0 and C) were substituted with peak’s area (S).
Specific energy input (SEI) was defined as follows:
where, P—MWP generator power supply (kW), V—volumetric gas flow rate (Nm3/h).
It should be explained that for the purpose of all the calculations an assumption was made that the volumetric gas flow rate did not change noticeably due to plasma treatment. Therefore, the inlet and outlet gas flow rate were equal. This assumption was partly proven during some of the test by the measurements of the gas flow rate with and without plasma working. It was also validated by the fact, that the gas composition did not change significantly due to plasma treatment and the dominating compound was nitrogen (see Table 3).
Results and Discussion
MWP Impact on the Permanent Compounds
Table 3 presents the analyses of the gas composition (considering permanent compounds) from both stages of the experiment. As it can be seen, the composition of the gas differs in both tests, which confirms that the work of the gasifier was not stable and not repeatable. Moreover, the gas obtained in the gasifier was of low quality, resulting in a low content of CO, H2, and CH4, and at the same time a high content of CO2. Nevertheless, the presented data quite clearly indicate the positive impact of MWP. The use of plasma resulted in a significant increase in the content of H2 and CO. The increase in the former was around 20–30%, although some doubts may occur in the case when the SEI of 1.14 kWh/Nm3 was applied. This result does not fit into the general trend of hydrogen content increase with the use of plasma (Table 3). This might have been caused by an error made during sampling or analysis, or from the unstable gasification process and temporary changes in the gas composition. There are no such ambiguities in the case of CO, since its concentration significantly increased in each run, and this increase ranged from 200 to 300%. Changes in the CO and H2 concentration were associated with a simultaneous decrease in CO2 and CH4 (as well as other hydrocarbons) yield. The CO2 may dissociate due to the high temperature of the plasma as in R1:
Moreover, carbon dioxide may interact with H2 (R2) and CH4 (R3) as well as with unprocessed char and soot (see R5) in Boudouard reaction (R4):
Methane (and other hydrocarbons) may simply decompose due to thermal dissociation (R5) but it may also react with H2O that is present in a producer gas (R6). It should be also mentioned that CH4 decomposition may be enhanced by the reaction with H radicals (R7).
It is also possible, for a water–gas shift reaction (R8) to take place in the lower part of the MWP reactor, where the temperature decreases:
A more detailed information considering the interaction between permanent compounds in the presence of MWP may be found in the previous work [28].
Analyzing the impact of plasma on light hydrocarbons present in the gas, it can be seen that methane and ethane show a clear decrease with the increasing SEI. In the case of other compounds, there is no such a clear trend, and their concentrations fluctuate around relatively stable values. With a multicomponent mixture, it is difficult to clearly indicate the reasons for this behavior. It should be noted that all of these compounds can decompose and, at the same time, create due to recombination of methane and other hydrocarbon decomposition products, as indicated in the literature [44, 45] and previous research of the authors [28].
In conclusion, the obtained data quite clearly demonstrate a positive effect of MWP on the quality of the gas, resulting in the increase of CO and H2 concentration at the cost of CO2 and hydrocarbons.
MWP Impact on the Aromatic Compounds
Table 4 shows the concentration of the main, quantified aromatic compounds that were identified in the producer gas and changes in the tar composition resulting from the plasma application. Besides the compounds shown in the table, the producer gas also contained other compounds that are graphically depicted in the chromatograms (Fig. 2a, b).
a Chromatograph from the 1st stage liquid samples. Black line—gas before MWP reactor, green line—gas after MWP reactor (SEI = 0.29 kWh/Nm3), red line—gas after MWP reactor (SEI = 0.59 kWh/Nm3), 1—benzene, 2—1,2-dimethylcyclopentane, 3—heptane, 4—methylcyclohexane, 5—2-methylheptane, 6—toluene, 7—1,4-dimethylcyclohexane, 8—octane, 9—ethylcyclohexane, 10—p-xylene, 11—styrene, 12—1,3-dimethylbenzene, 13—nonane, 14—propylbenzene, 15—1-ethyl-3-methylbenzene, 16—mesitylene, 17—decane, 18—indene, 19—naphthalene. b Chromatograph from the 2nd stage liquid samples. Black line—gas before MWP reactor, green line—gas after MWP reactor (SEI = 1.14 kWh/Nm3), red line—gas after MWP reactor (SEI = 1.44 kWh/Nm3), labels as in Fig. 3a except: 20—Benzothiophene. (Color figure online)
Analyzing the data in the table and chromatograms, it can be concluded that the producer gas consisted mainly of low-boiling organic compounds. These compounds included aromatics (mainly benzene and its substituted forms), cyclic compounds (substituted cyclopentane and cyclohexane compounds), and aliphatic compounds (C7–C10). The main, dominating compounds were benzene and toluene. At the same time, the gas also contained small concentrations of heavier compounds, i.e. indene, naphthalene and acenaphthylene.
Before considering the impact of plasma on the conversion of compounds contained in the gas, it should be noted that the individual tests differed not only in the SEI but also in the volumetric gas flow rate (see Table 2) as well as the initial concentrations of these compounds. While these facts make it difficult to directly and accurately compare the results, it does not affect the general trends and characteristics of the process.
Figure 3a, b provide the surface of the peaks of chosen tar compounds that were qualified in samples taken during the experiment. Additionally, the figures include conversion rate estimated on the basis of peak’s area (which is usually linearly proportional to concentration). Comparing these data with Table 4, it can be observed that a definitely higher conversion rate was achieved for cyclic compounds, aliphatic and substituted benzene compounds (including toluen ± e) than for the benzene itself. While the SEI of 0.29 kWh/Nm3 provides only a limited conversion rate (ranging from ca. 6 to 80% depending on the compound), the increase in Specific Energy Input up to 0.59 resulted in a significant improvement in the conversion rate (ranging from ca. 60 to 100% depending on the compound). In the case of higher SEI almost all tar compounds were completely converted. Only Styrene and p-Xylene achieved lower but still high conversion rate of 77–86% and 91–96%, respectively. The conversion rates obtained for toluene and benzene were lower, but their initial concentration was definitely higher. At the same time, gas conditioning using MWP also resulted in a noticeable increase in the concentration of indene, naphthalene, and acenaphthylene (Table 4). In general, it can be concluded that the increase in SEI resulted in an increase in the conversion rate of most compounds, but at the same time contributed to the increase in the share of heavier components such as indene, naphthalene, and acenaphthylene. However, the disproportion between the decomposed and created compounds should be noted—the decrease in the concentration of benzene and toluene was about two orders greater than the increase in heavier compounds.
a Peak’s area and simplified conversion rate of identified, but not quantified, compounds presented in liquid samples collected during 1st stage of experiment. b Peak’s area and simplified conversion rate of identified, but not quantified, compounds presented in liquid samples collected during 2nd stage of experiment
The observed influence of plasma on the changes in tar compounds composition is consistent with literature data and previous experiments. The relatively low degree of benzene conversion is related to the high thermal stability of this compound [21, 22, 40], the presence of light organic compounds that may recombine to benzene [28] and the possibility of benzene formation due to the decomposition of its substituted forms (like toluene [40]). Moreover, the decomposition products of benzene and other aromatics can lead to the formation of heavier compounds, including indene, naphthalene, and acenaphthylene. This process mainly involves reactions between phenyl radical (which is an important intermediate product of benzene decomposition) and light hydrocarbons and their radicals (mainly C2H2 but also C3 compounds)—eventually, this condensation process may lead to the production of soot [28, 40, 46]. In fact, thermal decomposition of benzene leading to the creation of phenyl radical (R9) and the following products seems natural in the context of MWP’s high temperature [40].
However, the addition of H2O, CO2, and H2 makes new reaction pathways available, due to the presence of O, OH and H radicals (R10, R11, R12) [47], thus enhancing the conversion rate [28, 40].
Moreover, these compounds, especially CO2 and H2O, strongly influence the final conversion product leading to the formation of CO and H2 rather than soot and hydrocarbons [28, 40]. Therefore, it may be stated that MWP enables tar compounds conversion due to high temperature and the presence of radicals. However, the radicals may be produced not only due to thermal dissociation but also thanks to vibrational excitation, which is typical for MWP and compounds like CO2, H2, and N2 [48, 49]. The direct influence of electrons or ions is rather negligible in the case of MWP due to low energy of the former [48] one and low concentration of the latter one [50]. More information considering MWP characteristics and its influence on tar compounds conversion may be found in the previous works [28, 40].
Comparison of the Simulated and the Sewage Sludge-Derived Producer Gas Results
The results presented in this paper are valuable due to the fact that they considered using MWP to clean a “real” produces gas obtained from the gasification process. However, this final step was preceded by an extensive research on a small laboratory scale with the use of model tar compounds and simulated gases [28, 40]. Referring to this lab-scale results and comparing them to those presented in this paper should provide a deeper insight into the process and allow pointing out any explicit differences.
Table 5 shows a composition of the simulated producer gas used in the previous research [28] and the producer gas obtained from sewage sludge gasification. The figure presents both the composition before and after plasma treatment. Since SEI is one of the most proper parameters allowing for the comparison of the process efficiency, two runs with the SEI closest to the “real” producer gas case (1.25 and 1.67 compared to 1.44 kW/Nm3) were chosen for the presentation of the simulated gas case results. Despite significant differences in the initial concentration of the producer gas components, the changes in the gas composition are consistent, leading to an increase in the H2 and CO concentration at the cost of CO2 and hydrocarbons. What is different is the scale of these changes. While the relative increase in H2 and CO is similar (20–30% in the case of H2 and 200–300% in the case of CO) the absolute is significantly smaller in the case of gasification derived producer gas. Since CO and H2 are produced from CO2 and hydrocarbons this drop in their absolute share can be clearly connected with the decrease in CH4 and CO2 conversion rate that characterize the “real” gas case.
Similar conversion drop can be attributed to benzene. Table 6 presents data considering benzene conversion (as a model tar compound or tar component) in both cases: the simulated lab-scale and the sewage sludge gasification experiments. Despite similar SEI, the lab-scale experiments showed a higher conversion rate. This is especially interesting since the higher conversion rate was achieved even though the initial benzene concentration was significantly higher (8.76 to ca. 0.9–1.8 g/Nm3). It can be pointed out that the gas derived from gasification included other hydrocarbons (including aromatics typical for tars). However, even if the concentration of benzene and toluene is summed up, it is far < 8.76 g/Nm3. The other hydrocarbons (see Fig. 3a, b) concentration is probably an order lower than the concentration of benzene (basing on the peak’s area). Moreover, these other compounds (including toluene), are converted much easier than benzene. It should be also mentioned, that the lab-scale experiments were characterized by a higher concentration of CH4 (compared to C1–C3 compounds concentration in the other case). It is important since the presence of methane (or any other light hydrocarbon) can significantly decrease benzene conversion rate due to the secondary creation of aromatics and competitive consumptions of radicals that are crucial in decomposition/conversion of hydrocarbons [28]. Summarizing, the sewage sludge gasification test showed a lower conversion rate of CO2 and hydrocarbons despite their lower initial concentration and similar SEI.
This phenomenon may have been a result of a few factors. Some influence may be assigned to the reactor’s geometry. Firstly, there were some differences in the space velocity comparing both reactors. In the case of simulated lab-scale experiments, the space velocity (calculated on the basis of normal conditions) was 0.64 s (for SEI = 1.66 kWh/Nm3) or 0.48 s (for SEI = 1.25 kWh/Nm3). In the second case (experiments with the producer gas), the space velocity was 0.61 s (SEI = 1.44 kWh/Nm3), 0.39 s (SEI = 1.14 kWh/Nm3), and 0.32 s (SEI = 0.29 and 0.59 kWh/Nm3). Therefore, it can be seen that in most runs in the “real” gas experiments, the space velocity was lower and so was the reaction time. However, it should be noted that the calculation of the space velocity did not include gas temperature or the fact that the gas flow was swirled. The second factor connected with the reactors’ geometry comes from a microwave energy distribution. In the case of simulated experiments, the reactor had only one generator focusing the whole microwave power (1.8 kW) in a small volume. The 10 kW reactor had four generators alongside the reactor’s quartz tube. Therefore, while the SEI might have been similar in both cases, the energy distribution has been quite different. In the second case, lower energy density might have resulted in a lower concentration of OH, O and H radicals which play a crucial role in organic compounds conversion [28, 40].
The stepwise distribution of the microwave energy alongside the quartz tube might have been also unfavorable due to another factor. During the experiments with producer gas, a residue layer was deposited on the inner walls. This deposit could have absorbed microwaves and inhibited their penetration through the gas. Interestingly, the deposit from the “real” gas experiments was quite different from the one obtained in the simulated gas experiments. The latter one was of purely organic origins. It was easy to remove from the quartz tube by blowing it off or washing. It was composed only of C, H, and N and its creation could have been easily and completely inhibited by the addition of CO2 or H2O [28, 40]. In the case of the experiments with the sewage sludge-derived producer gas, the deposit was hard to remove, and it was created even though the gas included CO2 and H2O. The SEM analyses proved that the deposit included, beside C, H, N and S, also elements like Al, Si, Fe, and O suggesting a partly inorganic origin of the deposit. Moreover, analysis of the experimental diffraction patterns (Fig. 4) allowed to state, that while the most of the deposit was in the form of amorphous, probably carbonous particles, crystalline phases as graphite and quartz, with the most intense peaks position similar to standards from JCPDS base (card numbers: 25-0284 and 33-1161, respectively), were also clearly identified. The presence of few additional peaks, nevertheless not very intensive, indicated the presence of other complex constituents in the deposit, corresponding to the standard cards no: 42-1491 (composed of Al, Si N and O). This is in contrary to the purely carbonous and amorphous structure of the soot obtained during the simulated lab-scale experiments [28]. The inorganic nature of the “real” gas experiments seems natural due to the high amount of ash in the sewage sludge (Table 1). In fact, the measurement of particles showed that their concentration was as high as 2.13–3.30 g/Nm3. This fact emphasizes another difference between the “real” and the simulated gas deposits. While the former one could have originated from the particles already present in the treated gas, the latter one was produced only due to the processes inside the plasma reactor. Consequently, conversion of the producer gas deposit involved heterogeneous reactions that could have been additionally limited by inorganic structures. In the case of simulated gas deposit, its creation could have been inhibited due to naturally faster homogenous reactions, i.e. between H2O/CO2 and soot precursors. Additional information considering the deposit might be also derived from the sewage sludge’s ash characteristic. Table 7 presents the composition of the ash and its characteristic temperatures. As it can be seen, the ash includes a lot of Si and Al what is consistent with the SEM and XRD analyses. Moreover, the softening and flowing temperatures of the ash are significantly below the temperatures that are obtained in the plasma reactor [28]. Therefore, it seems possible that the inorganic material in the producer gas was melted in the plasma core and deposited on the cooler walls of the quartz tube. The problem of deposit interfering with the microwaves transfer and plasma stability is a common issue in the MWP processing of carbon sources [42, 51].
Finally, another important factor that may have caused a difference in the conversion rate could have been connected with the hydrogen concentration. Hydrogen, or more specifically its H radicals (and derived OH radicals), has a positive effect on enhancing decomposition of hydrocarbons both aromatic (like benzene) and light ones (like methane). In the experiment with the producer gas, the H2 concentration was 2–3 times lower than in the case with the simulated gas experiments (Table 5). As a result, the influence of H radials might have been significantly limited. Additionally, the deposit on the quartz tube walls, inhibiting the microwave transfer, might have lowered the H population even more (as well as the temperature in the reactor). Consequently, the endothermic reaction R8 might have changed its direction (as in R13) and take part in decreasing the conversion rate [40].
Conclusion
Atmospheric MWP was applied as a method for producer gas processing. The producer gas was generated by sewage sludge gasification and partially cleaned via mechanical methods. Applying the MWP allowed to significantly reduce the concentration of residual tar compounds in the gas and positively influenced permanent gases composition.
The results showed that a higher conversion rate was achieved for substituted aromatics, as well as substituted and aliphatic hydrocarbons. A moderate conversion efficiency could have been attributed to benzene. Basing only on the quantified tar compounds, in the case of the highest SEI, the tar concentration was reduced from ca. 2453 to 643 mg/Nm3 (including benzene as a tar compound) or from ca. 620 to 125 mg/Nm3 (excluding benzene). As a result, the achieved conversion efficiency was ca. 73.8% or 79.9%, respectively. However, it should be indicated, that the real conversion rate was definitely higher since these values did not include other, unquantified tar compounds. While their concentration was minor in comparison to benzene or toluene, their conversion efficiency was much higher, often reaching 100%.
Besides tar compounds conversion, MWP treatment resulted in an increase in H2 and CO concentration due to the conversion of CO2 and hydrocarbons. This change in the composition may be especially attractive if the gas was to be used for synthesis or H2 production purpose.
Despite these advantages, the process might have some unwanted features. During the hydrocarbons conversion small amount of heavier aromatic, i.e. naphthalene, indene, and acenaphthylene were produced. However, the amount of these byproducts was disproportionately smaller (few mg/Nm3) than the amount of converted compounds (thousands of mg/Nm3). Another problematic issue was the formation of a char/inorganic deposit on the reactor’s walls. It is believed that this deposit limited the transfer of the microwave radiation into the quartz tube thus lowering the process efficiency and decreasing the conversion rate. This problem was mainly the result of the sewage sludge high ash content and its high melting and flowing temperatures.
Generally, the presented results and their comparison with the previous ones imply that the MWP technology may be considered as promising in terms of tar conversion and producer gas valorization. However, some further efforts are required to optimize the process, making it less energy consuming, limiting the heat losses and preventing formation of the deposit.
References
Ahmad, A.A., Zawawi, N.A., Kasim, F.H., Inayat, A., Khasri, A.: Assessing the gasification performance of biomass: a review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 53, 1333–1347 (2016). https://doi.org/10.1016/j.rser.2015.09.030
Kirkels, A.F., Verbong, G.P.J.: Biomass gasification: still promising? A 30-year global overview. Renew. Sustain. Energy Rev. 15, 471–481 (2011). https://doi.org/10.1016/j.rser.2010.09.046
Gadsbøll, R.Ø., Thomsen, J., Bang-Møller, C., Ahrenfeldt, J., Henriksen, U.B.: Solid oxide fuel cells powered by biomass gasification for high efficiency power generation. Energy 131, 198–206 (2017). https://doi.org/10.1016/j.energy.2017.05.044
Higman, C., van der Burgt, M.: Introduction. Gasification, 2nd edn. Gulf Professional Publishing, Houston (2008)
Asadullah, M.: Barriers of commercial power generation using biomass gasification gas: a review. Renew. Sustain. Energy Rev. 29, 201–215 (2014). https://doi.org/10.1016/j.rser.2013.08.074
Grachev, A., Zabelkin, S., Burenkov, S., Makarov, A., Bikbulatova, G., Pushkin, S., Zemskov, I.: Pyrolysis of fresh and deposited sewage sludge and investigation of the products. Waste Biomass Valoriz. (2017). https://doi.org/10.1007/s12649-017-0096-6
Werle, S., Wilk, R.K.: A review of methods for the thermal utilization of sewage sludge: the polish perspective. Renew. Energy 35, 1914–1919 (2010). https://doi.org/10.1016/j.renene.2010.01.019
Kelessidis, A., Stasinakis, A.S.: Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 32, 1186–1195 (2012). https://doi.org/10.1016/j.wasman.2012.01.012
Gil-Lalaguna, N., Sánchez, J.L., Murillo, M.B., Atienza-Martínez, M., Gea, G.: Energetic assessment of air-steam gasification of sewage sludge and of the integration of sewage sludge pyrolysis and air-steam gasification of char. Energy 76, 652–662 (2014). https://doi.org/10.1016/j.energy.2014.08.061
Freda, C., Cornacchia, G., Romanelli, A., Valerio, V., Grieco, M.: Sewage sludge gasification in a bench scale rotary kiln. Fuel 212, 88–94 (2018). https://doi.org/10.1016/j.fuel.2017.10.013
Kokalj, F., Arbiter, B., Samec, N.: Sewage sludge gasification as an alternative energy storage model. Energy Convers. Manag. 149, 738–747 (2017). https://doi.org/10.1016/j.enconman.2017.02.076
Striūgas, N., Valinčius, V., Pedišius, N., Poškas, R., Zakarauskas, K.: Investigation of sewage sludge treatment using air plasma assisted gasification. Waste Manag. 64, 149–160 (2017). https://doi.org/10.1016/j.wasman.2017.03.024
Werle, S.: Sewage sludge gasification process for clean and sustainable environment. Renew. Energy Environ. Sustain. 1, 35 (2016). https://doi.org/10.1051/rees/2016042
Milne, T.A., Evans, R.J.: Biomass gasifier “Tars”: their nature, formation, and conversion. Constraints (1998). https://doi.org/10.2172/3726
Anis, S., Zainal, Z.A.: Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: a review. Renew. Sustain. Energy Rev. 15, 2355–2377 (2011). https://doi.org/10.1016/j.rser.2011.02.018
Zwart, R.W.R., Van der Drift, A., Bos, A., Visser, H.J.M., Cieplik, M.K., Konemann, H.W.J.: Oil-based gas washing-flexible tar removal for high-efficient production of clean heat and power as well as sustainable fuels and chemicals. Environ. Prog. Sustain. Energy 28, 324–335 (2009)
Ud Din, Z., Zainal, Z.A.: Tar reduction mechanism via compression of producer gas. J. Clean. Prod. 184, 1–11 (2018). https://doi.org/10.1016/j.jclepro.2018.02.198
Fabry, F., Rehmet, C., Rohani, V., Fulcheri, L.: Waste gasification by thermal plasma: a review. Waste Biomass Valoriz. 4, 421–439 (2013). https://doi.org/10.1007/s12649-013-9201-7
Kwiecińska, A., Iluk, T., Kochel, M.: Utilization of aqueous-tar condensates formed during gasification. J. Ecol. Eng. 17, 132–137 (2016). https://doi.org/10.12911/22998993/65462
Knoef, H.A.M.: A review of syngas cleaning technologies. In: Knoef, H.A.M. (ed.) Handbook of Biomass Gasification, 2nd edn. BTG biomass technology group, Enschede (2012)
Jess, A.: Mechanisms and kinetics of thermal reactions of aromatic hydrocarbons from pyrolysis of solid fuels. Fuel 75, 1441–1448 (1996). https://doi.org/10.1016/0016-2361(96)00136-6
Jess, A.: Catalytic upgrading of tarry fuel gases: a kinetic study with model components. Chem. Eng. Process. Process Intensif. 35, 487–494 (1996). https://doi.org/10.1016/S0255-2701(96)04160-8
Chen, G., Li, J., Liu, C., Yan, B., Cheng, Z., Ma, W., Yao, J., Zhang, H.: Low-temperature catalytic cracking of biomass gasification tar over Ni/HZSM-5. Waste Biomass Valoriz. (2017). https://doi.org/10.1007/s12649-017-0107-7
Park, S.Y., Oh, G., Kim, K., Seo, M.W., Ra, H.W., Mun, T.Y., Lee, J.G., Yoon, S.J.: Deactivation characteristics of Ni and Ru catalysts in tar steam reforming. Renew. Energy 105, 76–83 (2017). https://doi.org/10.1016/j.renene.2016.12.045
Quitete, C.P.B., Manfro, R.L., Souza, M.M.V.M.: Perovskite-based catalysts for tar removal by steam reforming: effect of the presence of hydrogen sulfide. Int. J. Hydrogen Energy 42, 9873–9880 (2017). https://doi.org/10.1016/j.ijhydene.2017.02.187
Hongrapipat, J., Yip, A.C.K., Marshall, A.T., Saw, W.L., Pang, S.: Investigation of simultaneous removal of ammonia and hydrogen sulphide from producer gas in biomass gasification by titanomagnetite. Fuel 135, 235–242 (2014). https://doi.org/10.1016/j.fuel.2014.06.037
Pendyala, V.R.R., Gnanamani, M.K., Jacobs, G., Ma, W., Shafer, W.D., Davis, B.H.: Fischer-Tropsch synthesis: effect of ammonia impurities in syngas feed over a cobalt/alumina catalyst. Appl. Catal. A 468, 38–43 (2013). https://doi.org/10.1016/j.apcata.2013.07.060
Wnukowski, M., Jamróz, P.: Microwave plasma treatment of simulated biomass syngas: interactions between the permanent syngas compounds and their influence on the model tar compound conversion. Fuel Process. Technol. (2018). https://doi.org/10.1016/j.fuproc.2018.01.025
Wang, Y., Yang, H., Tu, X.: Plasma reforming of naphthalene as a tar model compound of biomass gasification. Energy Convers. Manag. 187, 593–604 (2019). https://doi.org/10.1016/j.enconman.2019.02.075
Liu, L., Zhang, Z., Das, S., Kawi, S.: Reforming of tar from biomass gasification in a hybrid catalysis-plasma system: a review. Appl. Catal. B 250, 250–272 (2019). https://doi.org/10.1016/j.apcatb.2019.03.039
Nair, S.A., Pemen, A.J.M., Yan, K., Van Gompel, F.M., Van Leuken, H.E.M., Van Heesch, E.J.M., Ptasinski, K.J., Drinkenburg, A.A.H.: Tar removal from biomass-derived fuel gas by pulsed corona discharges. Fuel Process. Technol. 84, 161–173 (2003). https://doi.org/10.1016/S0378-3820(03)00053-5
Pemen, A.J.M., Nair, S.A., Yan, K., Van Heesch, E.J.M., Ptasinski, K.J., Drinkenburg, A.A.H.: Pulsed corona discharges for tar removal from biomass derived fuel gas. Plasmas Polym. 8, 209–224 (2003). https://doi.org/10.1023/A:1024813306111
Liu, L., Wang, Q., Ahmad, S., Yang, X., Ji, M., Sun, Y.: Steam reforming of toluene as model biomass tar to H2-rich syngas in a DBD plasma-catalytic system. J. Energy Inst. (2017). https://doi.org/10.1016/j.joei.2017.09.003
Liu, S.Y., Mei, D.H., Nahil, M.A., Gadkari, S., Gu, S., Williams, P.T., Tu, X.: Hybrid plasma-catalytic steamreforming of toluene as a biomass tar model compound over Ni/Al2O3 catalysts. Fuel Process. Technol. 166, 669–675 (2017)
Materazzi, M., Lettieri, P., Mazzei, L., Taylor, R., Chapman, C.: Reforming of tars and organic sulphur compounds in a plasma-assisted process for waste gasification. Fuel Process. Technol. 137, 259–268 (2015). https://doi.org/10.1016/j.fuproc.2015.03.007
Chun, Y.N., Kim, S.C., Yoshikawa, K.: Decomposition of benzene as a surrogate tar in a gliding arc plasma. Environ. Prog. Sustain. Energy. 32, 837–845 (2013). https://doi.org/10.1002/ep.11663
Liu, S., Mei, D., Wang, L., Tu, X.: Steam reforming of toluene as biomass tar model compound in a gliding arc discharge reactor. Chem. Eng. J. 307, 793–802 (2017). https://doi.org/10.1016/j.cej.2016.08.005
Zhu, F., Li, X., Zhang, H., Wu, A., Yan, J., Ni, M., Zhang, H., Buekens, A.: Destruction of toluene by rotating gliding arc discharge. Fuel 176, 78–85 (2016). https://doi.org/10.1016/j.fuel.2016.02.065
Eliott, R.M., Nogueira, M.F.M., Silva Sobrinho, A.S., Couto, B.A.P., MacIel, H.S., Lacava, P.T.: Tar reforming under a microwave plasma torch. Energy Fuels 27, 1174–1181 (2013). https://doi.org/10.1021/ef301399q
Jamróz, P., Kordylewski, W., Wnukowski, M.: Microwave plasma application in decomposition and steam reforming of model tar compounds. Fuel Process. Technol. 169, 1–14 (2017). https://doi.org/10.1016/j.fuproc.2017.09.009
Medeiros, H.S., Pilatau, A., Nozhenko, O.S., Da Silva Sobrinho, A.S., Petraconi Filho, G.: Microwave air plasma applied to naphthalene thermal conversion. Energy Fuels 30, 1510–1516 (2016). https://doi.org/10.1021/acs.energyfuels.5b02451
Miotk, R., Hrycak, B., Czylkowski, D., Dors, M., Jasinski, M., Mizeraczyk, J.: Liquid fuel reforming using microwave plasma at atmospheric pressure. Plasma Sources Sci. Technol. (2016). https://doi.org/10.1088/0963-0252/25/3/035022
van Rooij, G.J., van den Bekerom, D.C.M., den Harder, N., Minea, T., Berden, G., Bongers, W.A., Engeln, R., Graswinckel, M.F., Zoethout, E., van de Sanden, M.C.M.: Taming microwave plasma to beat thermodynamics in CO2 dissociation. Faraday Discuss. 183, 233–248 (2015). https://doi.org/10.1039/C5FD00045A
Liu, C.J., Mallinson, R., Lobban, L.: Nonoxidative methane conversion to acetylene over zeolite in a low temperature plasma. J. Catal. 179, 326–334 (1998). https://doi.org/10.1006/jcat.1998.2225
Cuoci, A., Frassoldati, A., Faravelli, T., Ranzi, E.: A computational tool for the detailed kinetic modeling of laminar flames: application to C2H4/CH4 coflow flames. Combust. Flame 160, 870–886 (2013). https://doi.org/10.1016/j.combustflame.2013.01.011
Mebel, A.M., Landera, A., Kaiser, R.I.: Formation mechanisms of naphthalene and indene: from the interstellar medium to combustion flames. J. Phys. Chem. A 121, 901–926 (2017). https://doi.org/10.1021/acs.jpca.6b09735
Bittker, D.A.: Oxidation mechanisms of toluene and benzene. NASA Technical Paper 3546. National Aeronautics and Space Administration, Washington, DC (1995)
Dors, M., Nowakowska, H., Jasiński, M., Mizeraczyk, J.: Chemical kinetics of methane pyrolysis in microwave plasma at atmospheric pressure. Plasma Chem. Plasma Process. 34, 313–326 (2014). https://doi.org/10.1007/s11090-013-9510-4
Fridman, A.: Fundamentals of plasma chemistry: major components of chemically active plasma and mechanisms of plasma-chemical processes. Plasma Chemistry. Cambridge University Press, Cambridge (2008)
Pilatau, A., Medeiros, H.S., Da Silva Sobrinho, A.S., Petraconi Filho, G.: Numeric model for assessment of naphthalene conversion through ionization reactions in a microwave air plasma torch. Energy Fuels 30, 7704–7712 (2016). https://doi.org/10.1021/acs.energyfuels.6b01048
Minea, T., van den Bekerom, D.C.M., Peeters, F.J.J., Zoethout, E., Graswinckel, M.F., van de Sanden, M.C.M., Cents, T., Lefferts, L., van Rooij, G.J.: Non-oxidative methane coupling to C2 hydrocarbons in a microwave plasma reactor. Plasma Process. Polym. 15, 1–16 (2018). https://doi.org/10.1002/ppap.201800087
Acknowledgements
The results presented in this paper were obtained during the execution of the project implemented in the framework GEKON—Generator of the Ecological Concepts co-financed by the National Centre of Research and Development and the National Fund for Environmental Protection and Water Management by the Contract no. GEKON1/04/213768/32/2015—”Cogeneration system of sewage sludge gasification and gas cleaning using plasma”. Additionally, this work was supported by the Polish Ministry of Science and Higher Education (research task no 0402/0162/17).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wnukowski, M., Kordylewski, W., Łuszkiewicz, D. et al. Sewage Sludge-Derived Producer Gas Valorization with the Use of Atmospheric Microwave Plasma. Waste Biomass Valor 11, 4289–4303 (2020). https://doi.org/10.1007/s12649-019-00767-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00767-x