Abstract
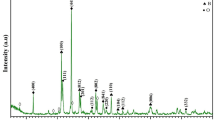
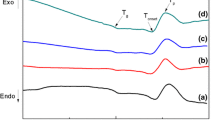
In this study, Fe-Al2O3-SiO2-P2O5 (FASP) glass–ceramic was prepared by heating Fe-acid-based geopolymer at 1050 °C. The Fe-geopolymer was obtained by mixing metakaolin and Fe filings with diluted phosphoric acid solution. X-Ray Diffraction (XRD) and Raman spectroscopies measurements were used to found the phase composition of the material. According to the results of XRD, the glass–ceramic contains AlSi2P3O12 and AlPO4 nanocrystals and an amorphous phase. With Raman spectroscopy we recognized the presence of AlPO4 and FeOx entities. Si–O-P, P-O-P bonds, and O = P double bonds are also identified. Glass–ceramic has been investigated for its semiconductor and linear and nonlinear optical properties. The variations of optical properties were further evaluated by UV–Visible and fluorescence spectroscopies. The chromaticity coordinates, and the fluorescence decay lifetime are recorded, analyzed and discussed. The nonlinear optical parameters are also determined and discussed. The FASP glass–ceramic exhibited an excellent UV–Visible absorption, a wide direct bandgap (3.72 eV), a distinguished fluorescence emission, a significant value of fluorescence decay lifetime 106 ns is showed. Thus, FASP glass–ceramic could have potential applications in solar cell and nonlinear optical devices.
Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
References
Sellami M, Barre M, Toumi M (2019) Synthesis, thermal properties and electrical conductivity of phosphoric acid-based geopolymer with metakaolin. Appl Clay Sci 180:105192. https://doi.org/10.1016/j.clay.2019.105192
Le-ping L, Xue-min C, Shu-heng Q et al (2010) Preparation of phosphoric acid-based porous geopolymers. Appl Clay Sci 50:600–603. https://doi.org/10.1016/j.clay.2010.10.004
Tchakouté HK, Rüscher CH, Kamseu E et al (2017) Influence of the molar concentration of phosphoric acid solution on the properties of metakaolin-phosphate-based geopolymer cements. Appl Clay Sci 147:184–194. https://doi.org/10.1016/j.clay.2017.07.036
Perera DS, Hanna JV, Davis J et al (2008) Relative strengths of phosphoric acid-reacted and alkali-reacted metakaolin materials. J Mater Sci 43:6562–6566. https://doi.org/10.1007/s10853-008-2913-6
Ma S, Zhang Z, Liu X (2022) Comprehensive understanding of aluminosilicate phosphate geopolymers: a critical review. Materials 15:5961. https://doi.org/10.3390/ma15175961
Yang X, Wu Y, Sun Z et al (2023) Preparation and properties of phosphoric acid-based porous geopolymer with high magnesium nickel slag and fly ash. Minerals 13:564. https://doi.org/10.3390/min13040564
Wu H, Yang J, Zhang Y et al (2023) Effects of spodumene flotation tailings on mechanical properties of acid-based geopolymer mortar. Minerals 13:150. https://doi.org/10.3390/min13020150
Petlitckaia S, Gharzouni A, Vlasceanu IN et al (2020) Effect of kaolin and argillite mixtures on the dielectric properties of geopolymers. Open Ceramics 4:100035. https://doi.org/10.1016/j.oceram.2020.100035
Wang F, Shao C, Yu C et al (2019) Effect of AlPO4 join concentration on optical properties and radiation hardening performance of Yb-doped Al2O3-P2O5-SiO2 glass. J Appl Phys 125. https://doi.org/10.1063/1.5096469
Rokita M, Handke M, Mozgawa W (1998) Spectroscopic studies of polymorphs of AlPO4 and SiO2. J Mol Struct 450:213–217. https://doi.org/10.1016/S0022-2860(98)00430-X
Handke M, Rokita M, Mozgawa W (1999) Spectroscopic studies of SiO2–AlPO4 solid solutions. Vib Spectrosc 19:419–423. https://doi.org/10.1016/S0924-2031(98)00083-6
Rokita M, Mozgawa W, Handke M (2001) The influence of Na+ and Ca2+ ions on the SiO2–AlPO4 materials structure — IR and Raman studies. J Mol Struct 596:171–178. https://doi.org/10.1016/S0022-2860(01)00708-6
Han L, Song J, Zhang Q et al (2018) Crystallization, structure and characterization of MgO-Al2O3-SiO2-P2O5 transparent glass-ceramics with high crystallinity. J Non-Cryst Solids 481:123–131. https://doi.org/10.1016/j.jnoncrysol.2017.10.028
Li CJ, Zhang YJ, Chen H et al (2022) Development of porous and reusable geopolymer adsorbents for dye wastewater treatment. J Clean Prod 348:131278. https://doi.org/10.1016/j.jclepro.2022.131278
Asim N, Alghoul M, Mohammad M et al (2019) Emerging sustainable solutions for depollution: geopolymers. Constr Build Mater 199:540–548. https://doi.org/10.1016/j.conbuildmat.2018.12.043
Falah M, MacKenzie KJD (2015) Synthesis and properties of novel photoactive composites of P25 titanium dioxide and copper (I) oxide with inorganic polymers. Ceram Int 41:13702–13708. https://doi.org/10.1016/j.ceramint.2015.07.198
Falah M, MacKenzie KJD, Knibbe R et al (2016) New composites of nanoparticle Cu (I) oxide and titania in a novel inorganic polymer (geopolymer) matrix for destruction of dyes and hazardous organic pollutants. J Hazard Mater 318:772–782. https://doi.org/10.1016/j.jhazmat.2016.06.016
Arokiasamy P, Abdullah MMAB, Abd Rahim SZ et al (2023) Diverse material based geopolymer towards heavy metals removal: a review. J Market Res 22:126–156. https://doi.org/10.1016/j.jmrt.2022.11.100
Shimizu E, Promentilla MA, Yu DE (2020) Utilization of coal fly ash and rice hull ash as geopolymer matrix-cum-metal dopant applied to visible-light-active nanotitania photocatalyst system for degradation of dye in wastewater. Catalysts 10:240. https://doi.org/10.3390/catal10020240
Falah M, MacKenzie KJD (2020) Photocatalytic nanocomposite materials based on inorganic polymers (geopolymers): a review. Catalysts 10:1158. https://doi.org/10.3390/catal10101158
Solati E, Dejam L, Dorranian D (2014) Effect of laser pulse energy and wavelength on the structure, morphology and optical properties of ZnO nanoparticles. Opt Laser Technol 58:26–32. https://doi.org/10.1016/j.optlastec.2013.10.031
Suryanarayana C, Norton MG (1998) X-ray diffraction a practical approach. New York: Plenum Press
Wang A, Freeman JJ, Jolliff BL (2015) Understanding the Raman spectral features of phyllosilicates. J Raman Spectrosc 46:829–845. https://doi.org/10.1002/jrs.4680
Gottardi V, Guglielmi M, Bertoluzza A et al (1984) Further investigations on Raman spectra of silica gel evolving toward glass. J Non-Cryst Solids 63:71–80. https://doi.org/10.1016/0022-3093(84)90387-9
Stolen RH, Walrafen GE (1976) Water and its relation to broken bond defects in fused silica. J Chem Phys 64:2623–2631. https://doi.org/10.1063/1.432516
Chiodini N, Meinardi F, Morazzoni F et al (1998) Tin doped silica by sol–gel method: doping effects on the SiO2 Raman spectrum. Solid State Commun 109:145–150. https://doi.org/10.1016/S0038-1098(98)00535-3
Spiekermann G, Steele-MacInnis M, Schmidt C, Jahn S (2012) Vibrational mode frequencies of silica species in SiO2-H2O liquids and glasses from ab initio molecular dynamics. J Chem Phys 136. https://doi.org/10.1063/1.3703667
Fischer M (2019) First-principles study of AlPO4-H3, a hydrated aluminophosphate zeotype containing two different types of adsorbed water molecules. Molecules 24:922. https://doi.org/10.3390/molecules24050922
Plotnichenko VG, Sokolov VO, Koltashev VV, Dianov EM (2002) On the structure of phosphosilicate glasses. J Non-Cryst Solids 306:209–226. https://doi.org/10.1016/S0022-3093(02)01172-9
Babita T, Anupam D, Govind PK, Pandey MK, Deb S (2007) Preparation and characterization of phosphate glasses containing titanium. https://api.semanticscholar.org/CorpusID:26885732
Rokita M, Handke M, Mozgawa W (2000) The AIPO4 polymorphs structure in the light of Raman and IR spectroscopy studies. J Mol Struct 555:351–356. https://doi.org/10.1016/S0022-2860(00)00620-7
Ettoumi H, Ben Ahmed A, Suñol JJ, Toumi M (2023) Effect of CuO added to aluminosilicate phosphate geopolymer, structure and optical properties analysis. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-023-02624-w
Le Saoût G, Simon P, Fayon F et al (2002) Raman and infrared study of (PbO) x (P 2 O 5) (1–x ) glasses. J Raman Spectrosc 33:740–746. https://doi.org/10.1002/jrs.911
Zotov N, Keppler H (2002) Silica speciation in aqueous fluids at high pressures and high temperatures. Chem Geol 184:71–82. https://doi.org/10.1016/S0009-2541(01)00353-9
Koo J, Bae B-S, Na H-K (1997) Raman spectroscopy of copper phosphate glasses. J Non-Cryst Solids 212:173–179. https://doi.org/10.1016/S0022-3093(96)00651-5
Kalampounias AG (2011) IR and Raman spectroscopic studies of sol–gel derived alkaline-earth silicate glasses. Bull Mater Sci 34:299–303. https://doi.org/10.1007/s12034-011-0064-x
Duan J, Ma B, Liu F et al (2018) Coordination ability determined transition metal ions substitution of Tb in Tb-Asp fluorescent nanocrystals and a facile ions-detection approach. Nanoscale 10:7526–7535. https://doi.org/10.1039/C7NR09267A
Goj P, Handke B, Stoch P (2022) Vibrational characteristics of aluminum–phosphate compounds by an experimental and theoretical approach. Sci Rep 12:17495. https://doi.org/10.1038/s41598-022-22432-5
Moguš-Milanković A, Šantić A, Reis ST et al (2005) Studies of lead–iron phosphate glasses by Raman, Mössbauer and impedance spectroscopy. J Non-Cryst Solids 351:3246–3258. https://doi.org/10.1016/j.jnoncrysol.2005.08.006
Stoch P, Szczerba W, Bodnar W et al (2014) Structural properties of iron-phosphate glasses: spectroscopic studies and ab initio simulations. Phys Chem Chem Phys 16:19917–19927. https://doi.org/10.1039/C4CP03113J
Souissi FZ, Ettoumi H, Barré M, Toumi M (2018) Preparation and electrical conductivity of potassium phosphate glasses containing Al2O3. J Non-Cryst Solids 481:585–589. https://doi.org/10.1016/j.jnoncrysol.2017.12.004
Baitahe R, Vittayakorn N, Boonchom B (2012) Study on thermal transformation of CuHPO4·H2O obtained by acetone-mediated synthesis at ambient temperature. J Therm Anal Calorim 110:625–632. https://doi.org/10.1007/s10973-011-1832-y
Yadav AK, Singh P (2015) A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv 5:67583–67609. https://doi.org/10.1039/C5RA13043C
Santos RM, Ling D, Sarvaramini A et al (2012) Stabilization of basic oxygen furnace slag by hot-stage carbonation treatment. Chem Eng J 203:239–250. https://doi.org/10.1016/j.cej.2012.06.155
Francis AA (2005) Non-isothermal crystallization kinetics of a blast furnace slag glass. J Am Ceram Soc 88:1859–1863. https://doi.org/10.1111/j.1551-2916.2005.00354.x
Francis AA (2006) Crystallization kinetics of magnetic glass–ceramics prepared by the processing of waste materials. Mater Res Bull 41:1146–1154. https://doi.org/10.1016/j.materresbull.2005.11.002
Agathopoulos S, Tulyaganov DU, Ventura JMG et al (2006) Structural analysis and devitrification of glasses based on the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. J Non-Cryst Solids 352:322–328. https://doi.org/10.1016/j.jnoncrysol.2005.12.003
Osipov AA, Osipova LM, Eremyashev VE (2013) Structure of alkali borosilicate glasses and melts according to Raman spectroscopy data. Glass Phys Chem 39:105–112. https://doi.org/10.1134/S1087659613020119
Koroleva ON, Shabunina LA, Bykov VN (2011) Structure of borosilicate glass according to raman spectroscopy data. Glass Ceram 67:340–342. https://doi.org/10.1007/s10717-011-9293-0
Goel A, McCloy JS, Windisch CF et al (2013) Structure of rhenium-containing sodium borosilicate glass. Int J Appl Glas Sci 4:42–52. https://doi.org/10.1111/ijag.12003
Barrio RA, Galeener FL, Martínez E, Elliott RJ (1993) Regular ring dynamics in AX2 tetrahedral glasses. Phys Rev B 48:15672–15689. https://doi.org/10.1103/PhysRevB.48.15672
de Bonfils J, Peuget S, Panczer G et al (2010) Effect of chemical composition on borosilicate glass behavior under irradiation. J Non-Cryst Solids 356:388–393. https://doi.org/10.1016/j.jnoncrysol.2009.11.030
Matson DW, Sharma SK, Philpotts JA (1983) The structure of high-silica alkali-silicate glasses. A Raman spectroscopic investigation. J Non-Cryst Solids 58:323–352. https://doi.org/10.1016/0022-3093(83)90032-7
Galeener FL (1982) Planar rings in vitreous silica. J Non-Cryst Solids 49:53–62. https://doi.org/10.1016/0022-3093(82)90108-9
Zhang R, Min Y, Wang Y et al (2020) Structural evolution of molten slag during the early stage of basic oxygen steelmaking. ISIJ Int 60:212–219. https://doi.org/10.2355/isijinternational.ISIJINT-2019-413
Babenko AA, Shartdinov RR, Upolovnikova AG, Smetannikov AN (2023) Effects of basicity on the phase composition, structure, viscosity, and crystallization temperature of CaO–SiO2–Al2O3–MgO–B2O3 slags. Metallurgist 67:166–175. https://doi.org/10.1007/s11015-023-01499-z
Lucazeau G, Sergent N, Pagnier T et al (2007) Raman spectra of apatites: La 10–x Si 6− y (Al, Fe) y O 26±δ. J Raman Spectrosc 38:21–33. https://doi.org/10.1002/jrs.1569
Iordanova R, Dimitriev Y, Dimitrov V, Klissurski D (1994) Structure of V2O5-MoO3-Fe2O3 glasses. J Non-Cryst Solids 167:74–80. https://doi.org/10.1016/0022-3093(94)90369-7
Testa-Anta M, Ramos-Docampo MA, Comesaña-Hermo M et al (2019) Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Advances 1:2086–2103. https://doi.org/10.1039/C9NA00064J
Di Genova D, Sicola S, Romano C et al (2017) Effect of iron and nanolites on Raman spectra of volcanic glasses: a reassessment of existing strategies to estimate the water content. Chem Geol 475:76–86. https://doi.org/10.1016/j.chemgeo.2017.10.035
Di Genova D, Morgavi D, Hess K et al (2015) Approximate chemical analysis of volcanic glasses using Raman spectroscopy. J Raman Spectrosc 46:1235–1244. https://doi.org/10.1002/jrs.4751
Di Muro A, Métrich N, Mercier M et al (2009) Micro-Raman determination of iron redox state in dry natural glasses: application to peralkaline rhyolites and basalts. Chem Geol 259:78–88. https://doi.org/10.1016/j.chemgeo.2008.08.013
Wang ZJ, Shu QF, Sridhar S et al (2015) Effect of P2O5 and FetO on the viscosity and slag structure in steelmaking slags. Metall Mater Trans B 46:758–765. https://doi.org/10.1007/s11663-014-0270-1
Chamritski I, Burns G (2005) Infrared- and Raman-active phonons of magnetite, maghemite, and hematite: a computer simulation and spectroscopic study. J Phys Chem B 109:4965–4968. https://doi.org/10.1021/jp048748h
de Faria DLA, Venâncio Silva S, de Oliveira MT (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc 28:873–878. https://doi.org/10.1002/(SICI)1097-4555(199711)28:11%3c873::AID-JRS177%3e3.0.CO;2-B
Trukhin A, Capoen B (2005) Raman and optical reflection spectra of germanate and silicate glasses. J Non-Cryst Solids 351:3640–3643. https://doi.org/10.1016/j.jnoncrysol.2005.09.017
Grosso-Giordano NA, Yeh AJ, Okrut A et al (2017) Effect of defect site preorganization on Fe(III) grafting and stability: a comparative study of delaminated zeolite vs amorphous silica supports. Chem Mater 29:6480–6492. https://doi.org/10.1021/acs.chemmater.7b02062
Abdelghany AM, ElBatal HA (2014) Gamma-rays interactions on optical, FTIR absorption and ESR spectra of 3d transition metals-doped sodium silicophosphate glasses. J Mol Struct 1067:138–146. https://doi.org/10.1016/j.molstruc.2014.03.032
Abdelghany AM, Zeyada HM, ElBatal HA, Fetouh R (2016) Synthesis and spectral properties of Nd2O3-doped sodium silicophosphate glass. SILICON 8:325–330. https://doi.org/10.1007/s12633-015-9308-5
Abdelghany AM, ElBatal HA (2012) Structural evaluation and shielding behavior of gamma irradiated vanadium doped silicophosphate glasses. J Mol Struct 1024:47–53. https://doi.org/10.1016/j.molstruc.2012.05.038
Arakha M, Saleem M, Mallick BC, Jha S (2015) The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci Rep 5:9578. https://doi.org/10.1038/srep09578
Reddy RR, Rama Gopal K, Narasimhulu K et al (2008) Correlation between optical electronegativity and refractive index of ternary chalcopyrites, semiconductors, insulators, oxides and alkali halides. Opt Mater 31:209–212. https://doi.org/10.1016/j.optmat.2008.03.010
Jilani A, Abdel-wahab MS, Al-ghamdi AA et al (2016) Nonlinear optical parameters of nanocrystalline AZO thin film measured at different substrate temperatures. Physica B 481:97–103. https://doi.org/10.1016/j.physb.2015.10.038
Frumar M, Jedelský J, Frumarová B et al (2003) Optically and thermally induced changes of structure, linear and non-linear optical properties of chalcogenides thin films. J Non-Cryst Solids 326–327:399–404. https://doi.org/10.1016/S0022-3093(03)00446-0
Xia J, Liu Y, Qiu X et al (2012) Solvothermal synthesis of nanostructured CuInS2 thin films on FTO substrates and their photoelectrochemical properties. Mater Chem Phys 136:823–830. https://doi.org/10.1016/j.matchemphys.2012.08.005
Abutalib MM, Yahia IS (2019) Analysis of the linear/nonlinear optical properties of basic fuchsin dye/FTO films: controlling the laser power of red/green lasers. Optik 179:145–153. https://doi.org/10.1016/j.ijleo.2018.10.081
Mohammedi A, Ibrir M, Meglali O, Berri S (2021) Influence of Cu‐doping on linear and nonlinear optical properties of high‐quality ZnO thin films obtained by spin‐coating technique. Phys Status Solidi (B) 258. https://doi.org/10.1002/pssb.202000472
Abomostafa HM (2021) Linear and nonlinear optical properties of innovative synthesis of nickel nanoparticles in polystyrene matrix as a new optical system. J Mol Struct 1225:129126. https://doi.org/10.1016/j.molstruc.2020.129126
Qasem A, Hassaan MY, Moustafa MG et al (2020) Optical and electronic properties for As-60 at.% S uniform thickness of thin films: influence of Se content. Opt Mater 109:110257. https://doi.org/10.1016/j.optmat.2020.110257
Sahoo D, Priyadarshini P, Aparimita A et al (2021) Optimization of linear and nonlinear optical parameters of As40Se50Te10 thin films by thermal annealing. Opt Laser Technol 140:107036. https://doi.org/10.1016/j.optlastec.2021.107036
Sahoo D, Priyadarshini P, Dandela R et al (2021) In situ laser irradiation: the kinetics of the changes in the nonlinear/linear optical parameters of As 50 Se 40 Sb 10 thin films for photonic applications. RSC Adv 11:16015–16025. https://doi.org/10.1039/D1RA02368C
Shaaban ER, Hassaan MY, Moustafa MG et al (2019) Optical constants, dispersion parameters and non-linearity of different thickness of As40S45Se15 thin films for optoelectronic applications. Optik 186:275–287. https://doi.org/10.1016/j.ijleo.2019.04.097
Mansour AM, Abou Hammad AB, Bakr AM, El Nahrawy AM (2022) Silica zinc titanate wide bandgap semiconductor nanocrystallites: synthesis and characterization. SILICON 14:11715–11729. https://doi.org/10.1007/s12633-022-01886-2
Jamalaiah BC, Khan PS, Saloni et al (2023) Sr3Gd(PO4)3: Dy3+ phosphors for lighting applications. J Sol-Gel Sci Technol 105:266–277. https://doi.org/10.1007/s10971-022-05995-7
Patle Y, Brahme N, Bisen DP et al (2021) Study of Photoluminescence, Thermoluminescence, and Afterglow properties of Dy3+ doped Ba2ZnSi2O7 phosphor. Optik 226:165896. https://doi.org/10.1016/j.ijleo.2020.165896
Bedyal AK, Kumar V, Swart HC (2020) Influence of an adjoining cation on the luminescence performance of the Dy3+ doped A3Gd(PO4)2; (A= Na, K) phosphors. J Alloy Compd 845:156352. https://doi.org/10.1016/j.jallcom.2020.156352
Wang Z-Y, Shen B-L, Yu K-H et al (2019) Tunable white-light emission of Dy3+ or/and Eu3+ co-doped single-phase LiY(PO3)4 phosphors for NUV-WLEDs. J Alloy Compd 791:833–838. https://doi.org/10.1016/j.jallcom.2019.03.406
Li Y, Chen J, Chen C (2018) Tunable correlated color temperature of NaSrPO4 phosphors via Dy3+ and Eu3+ co-doping for warm white light-emitting diodes. Optik 174:1–6. https://doi.org/10.1016/j.ijleo.2018.08.055
Haritha P, Martín IR, Dwaraka Viswanath CS et al (2017) Structure, morphology and optical characterization of Dy 3+ -doped BaYF 5 nanocrystals for warm white light emitting devices. Opt Mater 70:16–24. https://doi.org/10.1016/j.optmat.2017.05.002
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors (Houda Ettoumi, Ali Ben Ahmed, Mohamed Toumi) and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the corresponding author “Ali Ben Ahmed” is the sole contact for the editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
All authors contributed equally to this paper:
1- Houda Ettoumi: Methodology, Data curation, conceptualization, first draft writing.
2- Ali Ben Ahmed: Investigation, Data curation, Writing and Reviewing, and editing.
3- Mohamed Toumi: Spectroscopies measurement, Validation, formal analysis.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ettoumi, H., Ben Ahmed, A. & Toumi, M. Preparation and Characterization of Fe-Containing Aluminosilicate Phosphate Ceramic-Glass from a Geopolymer Precursor: Insights from XRD, Raman Spectroscopy, and Optical Properties. Silicon (2024). https://doi.org/10.1007/s12633-023-02844-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12633-023-02844-2