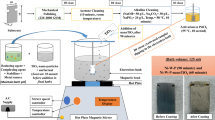
Abstract
The present study aims to investigate the thermophysical behavior of shielded metal arc welding (SMAW) designed by using CaO-SiO2-TiO2-MgO systems for offshore applications. An extreme vertices design approach is used to formulate twenty-six SMAW electrode coatings. Thermophysical properties such as thermal conductivity, thermal diffusivity, and specific heat of each coating powder were evaluated by the Hot-Disc apparatus. The analysis of weight loss & enthalpy was performed using a thermogravimetric analyzer (TGA). The structure and phases of the coating material were investigated using the X-ray diffraction (XRD) technique. Fourier Transform Infrared (FTIR) Spectroscopy technique has been used for the structural characterization of shielded metal arc welding SMAW electrode coatings powder. By using various regression models, individual, binary, and ternary coating constituents have been developed for physicochemical & thermophysical properties. Regression study has shown that whereas binary interaction increases the thermal conductivity of electrode coating, individual constituents have a decreasing influence. On the thermal diffusivity of the electrode coating, individual constituents have a synergistic effect whereas binary constituents have an anti-synergistic effect. While binary interactions have an increasing effect, MgO is the only individual component that has a synergistic effect on weight loss. The binary mixture CaO.TiO2, CaO.MgO, SiO2.MgO, and TiO2.MgO has a synergistic influence on enthalpy, but SiO2 is the sole individual constituent to do so. The generated artificial neural network (ANN) models are evaluated for prediction accuracy and contrasted using regression analysis.
Similar content being viewed by others
Data Availability
I, Sudish Mishra, confirm that the data and materials will be made available upon request from the author.
References
Mvola B, Kah P, Martikainen J, Suoranta R (2016) Dissimilar welded joints operating in sub-zero temperature environment. Int J Adv Manuf Technol 87:3619–3635. https://doi.org/10.1007/s00170-016-8711-4
Olsson J, Snis M (2007) Duplex - a new generation of stainless steels for desalination plants. Desalination 205:104–113. https://doi.org/10.1016/j.desal.2006.02.051
Sadeghian M, Shamanian M, Shafyei A (2014) Effect of heat input on microstructure and mechanical properties of dissimilar joints between super duplex stainless steel and high strength low alloy steel. Mater Des 60:678–684. https://doi.org/10.1016/j.matdes.2014.03.057
Belkessa B, Miroud D, Ouali N, Cheniti B (2016) Microstructure and mechanical behavior in dissimilar SAF 2205/API X52 welded pipes. Acta Metall Sin (Engl Lett) 29:674–682. https://doi.org/10.1007/s40195-016-0428-8
Wang S, Ma Q, Li Y (2011) Characterization of microstructure, mechanical properties and corrosion resistance of dissimilar welded joint between 2205 duplex stainless steel and 16MnR. Mater Des 32:831–837. https://doi.org/10.1016/j.matdes.2010.07.012
Sarlak H, Atapour M, Esmailzadeh M (2015) Corrosion behavior of friction stir welded lean duplex stainless steel. Mater Des 66:209–216. https://doi.org/10.1016/j.matdes.2014.10.060
López-Ortega A, Bayón R, Arana JL et al (2018) Influence of temperature on the corrosion and tribocorrosion behaviour of high-strength low-alloy steels used in offshore applications. Tribol Int 121:341–352. https://doi.org/10.1016/j.triboint.2018.01.049
Rosliza R, Wan Nik WB (2010) Improvement of corrosion resistance of AA6061 alloy by tapioca starch in seawater. Curr Appl Phys 10:221–229. https://doi.org/10.1016/j.cap.2009.05.027
Ezuber H, El-Houd A, El-Shawesh F (2008) A study on the corrosion behavior of aluminum alloys in seawater. Mater Des 29:801–805. https://doi.org/10.1016/j.matdes.2007.01.021
Dutra JC, e Silva RHG, Savi BM, et al (2015) Metallurgical characterization of the 5083H116 aluminum alloy welded with the cold metal transfer process and two different wire-electrodes (5183 and 5087). Weld World 59:797–807. https://doi.org/10.1007/s40194-015-0253-0
Yan C, Zeng Q, Xu Y, He W (2019) Microstructure, phase and tribocorrosion behavior of 60NiTi alloy. Appl Surf Sci 498:143838. https://doi.org/10.1016/j.apsusc.2019.143838
Sharma L, Chhibber R, Kumar V, Khan WN (2023) Element transfer investigations on silica based submerged arc welding fluxes. Silicon 15:305–319. https://doi.org/10.1007/s12633-022-02004-y
Jena G, Anandkumar B, Vanithakumari SC et al (2020) Graphene oxide-chitosan-silver composite coating on Cu-Ni alloy with enhanced anticorrosive and antibacterial properties suitable for marine applications. Prog Org Coat 139:105444. https://doi.org/10.1016/j.porgcoat.2019.105444
Yan S, Song GL, Li Z et al (2018) A state-of-the-art review on passivation and biofouling of Ti and its alloys in marine environments. J Mater Sci Technol 34:421–435. https://doi.org/10.1016/j.jmst.2017.11.021
Gurrappa I (2003) Characterization of titanium alloy Ti-6Al-4V for chemical, marine and industrial applications. Mater Charact 51:131–139. https://doi.org/10.1016/j.matchar.2003.10.006
Maurya AK, Pandey C, Chhibber R (2021) Dissimilar welding of duplex stainless steel with Ni alloys: a review. Int J Press Vess Piping 192:104439. https://doi.org/10.1016/j.ijpvp.2021.104439
Barnhouse EJ, Lippold JC (1998) Microstructure/property relationships in dissimilar welds between duplex stainless steels and carbon steels. WeldJ 77:477-s
Rathod DW, Pandey S, Singh PK, Prasad R (2015) A experimental analysis of dissimilar metal weld joint : ferritic to austenitic stainless steel. Mater Sci Eng A 639:259–268. https://doi.org/10.1016/j.msea.2015.05.011
Akram J, Kalvala PR, Chalavadi P, Misra M (2018) Dissimilar metal weld joints of P91/Ni alloy: microstructural characterization of HAZ of P91 and stress analysis at the weld interfaces. J Mater Eng Perform 27:4115–4128. https://doi.org/10.1007/s11665-018-3502-8
DuPont JN (2012) Microstructural evolution and high temperature failure of ferritic to austenitic dissimilar welds. Int Mater Rev 57:208–234. https://doi.org/10.1179/1743280412Y.0000000006
Shah Hosseini H, Shamanian M, Kermanpur A (2011) Characterization of microstructures and mechanical properties of Inconel 617/310 stainless steel dissimilar welds. Mater Charact 62:425–431. https://doi.org/10.1016/j.matchar.2011.02.003
Chhibber R, Arora N, Gupta SR, Dutta BK (2006) Use of bimetallic welds in nuclear reactors: associated problems and structural integrity assessment issues. Proc Inst Mech Eng C J Mech Eng Sci 220:1121–1133. https://doi.org/10.1243/09544062JMES135
Sharma L, Chhibber R (2019) Design of TiO2-SiO2-MgO and SiO2-MgO-Al2O3-based submerged arc fluxes for multipass bead on plate pipeline steel welds. J Press Vessel Technol Trans ASME 141. https://doi.org/10.1115/1.4043375
Jindal S, Chhibber R, Mehta NP (2013) Investigation on flux design for submerged arc welding of high-strength low-alloy steel. Proc Inst Mech Eng B J Eng Manuf 227:383–395. https://doi.org/10.1177/0954405412468993
Eagar (1978) Sources of weld metal oxygen contaminiation during SAW. Weld J (Miami, Fla) 57:76–80
Natalie CA, Olson DL, Blander M (1986) Physical and chemical behavior of welding fluxes. Annu Rev Mater Sci 16(1):389–413
Qin R, He G (2013) Mass transfer of nickel-base alloy covered electrode during shielded metal arc welding. Metall Mater Trans A Phys Metall Mater Sci 44:1475–1484. https://doi.org/10.1007/s11661-012-1505-x
Bhandari D, Chhibber R, Arora N (2016) Investigations on weld metal chemistry and mechanical behaviour of bimetallic welds using CaO–CaF2–SiO2–Ni based electrode coatings. 0:1–17. https://doi.org/10.1177/1464420716677316
Wang H, Qin R, He G (2016) SiO2 and CaF2 Behavior During Shielded Metal Arc Welding and Their Effect on Slag Detachability of the CaO-CaF2-SiO2 Type ENiCrFe-7-Covered Electrode. Metall Mater Trans A Phys Metall Mater Sci 47:4530–4542. https://doi.org/10.1007/s11661-016-3629-x
Sharma L, Chhibber R (2020) Investigations of thermophysical properties of submerged arc welding slag using a rutile-acidic flux system. CIRP J Manuf Sci Technol 31:322–333. https://doi.org/10.1016/j.cirpj.2020.06.006
Kumar V, Kumar J, Chhibber R, Sharma L (2022) Experimental study on wettability at high-temperature using TiO2-SiO2-CaO-Na3AlF6 based electrode coating for AUSC thermal power plant. Silicon. 1–13
Khan WN, Chhibber R (2021) Investigations on effect of CaO-CaF2-TiO2-SiO2 based electrode coating constituents and their interactions on weld chemistry. Ceram Int 47:12483–12493. https://doi.org/10.1016/j.ceramint.2021.01.106
Mahajan S, Chhibber R (2020) Investigation on slags of CaO-CaF2-SiO2- Al2O3 based electrode coatings developed for power plant welds. Ceram Int. https://doi.org/10.1016/j.ceramint.2019.12.117
Kumar V, Chhibber R (2022) Physicochemical and thermophysical properties of CaO–TiO2–SiO2–Na3AlF6 system based electrode coating for AUSC power plant. Ceram Int 48:17412–17424. https://doi.org/10.1016/j.ceramint.2022.03.005
Mahajan S, Sharma L, Chhibber R (2022) Effect of CaO-CaF2-SiO2-Al2O3 Based Electrode Coating Constituents and their Interactions on the P22 Alloy SMAW Dissimilar Weld Metal delta Quantities. Silicon 12315–12327. https://doi.org/10.1007/s12633-022-01937-8
Gupta A, Singh J, Chhibber R (2022) Investigation of thermophysical and physicochemical characteristics of Al2O3-SiO2-CaO-Na3AlF6 flux for SMAW electrode coating. Silicon 1–15.
Mishra S, Sharma L, Chhibber R (2023) Experimental investigation on thermo-physical behavior of basic electrode coatings for offshore applications. Silicon. https://doi.org/10.1007/s12633-023-02291-z
Kumar V, Chhibber R, Sharma L (2023) Investigation on Thermophysical and Physicochemical Properties of CaO-SiO2-CaF2-22.5%TiO2 Silica Based Electrode Coating System. Silicon 15:739–753. https://doi.org/10.1007/s12633-022-02037-3
Kumar V, Kumar J, Chhibber R, Sharma L (2023) Investigation on CaO-SiO2-CaF2-SrO Based Electrode Coating System on High-Temperature Wettability and Structural Behaviour for Power Plants Welds. Silicon 15:1933–1946. https://doi.org/10.1007/s12633-022-02145-0
Mishra S, Sharma L, Chhibber R (2023) Investigation on Wettability Behaviour of Electrode Coatings using Silica-based SiO2-CaO-CaF2 and ZrO2-CaO-SiO2 Flux System. Silicon. https://doi.org/10.1007/s12633-023-02434-2
Bhandari D, Chhibber R, Arora N, Mehta R (2019) Investigations on weld metal chemistry and mechanical behaviour of bimetallic welds using CaO–CaF2–SiO2–Ni based electrode coatings. Proc Inst Mech Eng Part L: J Mater: Des Appl 233:563–579. https://doi.org/10.1177/1464420716677316
Kim JH, Frost RH, Olson DL, Blander M (1990) Effect of electrochemical reactions on submerged arc weld metal compositions. WeldJ 69(12):446–453
Mukerji J (1965) Phase Equilibrium Diagram CaO—CaF2—2CaO SiO2. J Am Ceram Soc 48(4):210–213
Eriksson G, Pelton AD (1993) Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CaO-Al2O3, Al2O3-SiO2, and CaO-Al2O3-SiO2 systems. Metall Trans B 24:807–816. https://doi.org/10.1007/BF02663141
Sridhar S, Mills KC, Afrange ODC, Lörz HP, Carli R (2000) Break temperatures of mould fluxes and their relevance to continuous casting. Ironmak Steelmak 27(3):238–242. https://doi.org/10.1179/030192300677534
Kanjilal P, Pal TK, Majumdar SK (2006) Combined effect of flux and welding parameters on chemical composition and mechanical properties of submerged arc weld metal. J Mater Process Technol 171:223–231. https://doi.org/10.1016/j.jmatprotec.2005.06.083
McLean RA, Anderson VL (1966) Extreme Vertices Design of Mixture Experiments. Technometrics 8:447–454. https://doi.org/10.1080/00401706.1966.10490377
Sharma L (2018) Design and development of submerged arc welding fluxes using TiO 2 -SiO 2 -CaO. Proc Inst Mech Eng Par E J Proc Mech Eng 0:1–24. https://doi.org/10.1177/0954408918794036
Khan WN, Chhibber R (2020) Physicochemical and thermo physical characterization of CaO–CaF2–SiO2 and CaO–TiO2–SiO2 based electrode coating for offshore welds. Ceram Int 46:8601–8614. https://doi.org/10.1016/j.ceramint.2019.12.092
Ricker RW, Osborn EF (1954) Additional Phase Equilibrium Data for the System CaO-MgO-SiO2. J Am Ceram Soc 37:133–139. https://doi.org/10.1111/j.1151-2916.1954.tb14011.x
Roy R, Osborn EF (1954) CaO -TiO,-SiO,. 38:158–171
Gasparik T (2003) Phase Diagrams for Geoscientists. Berlin, Heidelberg. Springer Berlin Heidelberg
El-Sadek MH, El-Barawy K, Morsi IM (2019) Production of calcium metal by aluminothermic reduction of Egyptian limestone ore. Can Metall Q 58:213–222. https://doi.org/10.1080/00084433.2018.1544343
Nouar SL, Djebaili Baki A, Iddou A (2007) Studies of the mechanism of poly (vinyl alcohol) (PVA) adsorption on the calcite/water interface in the presence of sodium oleate (SOL). Res J Chem Environ 11:52–62. https://doi.org/10.4236/jmmce.2008.72012
Alharbi ND (2015) Size controlled CaFnanocubes and their dosimetric properties using photoluminescence technique. J Nanomater 2015:1–8. https://doi.org/10.1155/2015/136957
Sharma L, Chhibber R (2019) Investigating the physicochemical and thermophysical properties of submerged arc welding fluxes designed using TiO2-SiO2-MgO and SiO2-MgO-Al2O3 flux systems for linepipe steels. Ceram Int 45:1569–1587. https://doi.org/10.1016/j.ceramint.2018.10.032
Kaur G, Kumar M, Arora A et al (2011) Influence of Y2O3 on structural and optical properties of SiO2-BaO-ZnO-xB2O3-(10–x) Y 2O3 glasses and glass ceramics. J Non Cryst Solids 357:858–863. https://doi.org/10.1016/j.jnoncrysol.2010.11.103
Garai M, Sasmal N, Molla AR et al (2015) Effects of in-situ generated coinage nanometals on crystallization and microstructure of fluorophlogopite mica containing glass-Ceramics. J Mater Sci Technol 31:110–119. https://doi.org/10.1016/j.jmst.2014.11.012
Garai M, Sasmal N, Molla AR et al (2014) Effects of nucleating agents on crystallization and microstructure of fluorophlogopite mica-containing glass-ceramics. J Mater Sci 49:1612–1623. https://doi.org/10.1007/s10853-013-7844-1
Sowmya T, Sankaranarayanan SR (2004) Spectroscopic analysis of slags preliminary observations. VII Int Conf on Molten Slags Fluxes and Salts 693–698.
Sharma L, Kumar J, Chhibber R (2020) Experimental investigation on high temperature wettability and structural behaviour of SAW fluxes using MgO–TiO2–SiO2 and Al2O3–MgO–SiO2 flux system. Ceram Int 46:5649–5657. https://doi.org/10.1016/j.ceramint.2019.11.011
Khan WN, Chhibber R (2021) Characterization of CaO-CaF2-TiO2-SiO2 Based Welding Slags for Physicochemical and Thermophysical Properties. Silicon 13:1575–1589. https://doi.org/10.1007/s12633-020-00537-8
Kerstan M, Müller M, Rüssel C (2011) Binary, ternary and quaternary silicates of CaO, BaO and ZnO in high thermal expansion seals for solid oxide fuel cells studied by high-temperature X-ray diffraction (HT-XRD). Mater Res Bull 46:2456–2463. https://doi.org/10.1016/j.materresbull.2011.08.031
Mills KC (2011) The Estimation Of Slag Properties. Southern Afr Pyrometall 2011 Int Conf. 1–52.
Kang Y, Lee J, Morita K (2014) Thermal conductivity of molten slags: A review of measurement techniques and discussion based on microstructural analysis. ISIJ Int 54:2008–2016. https://doi.org/10.2355/isijinternational.54.2008
Li Y, Yu B, Wang B et al (2020) Online quality inspection of ultrasonic composite welding by combining artificial intelligence technologies with welding process signatures. Mater Des 194:108912. https://doi.org/10.1016/j.matdes.2020.108912
Tian Z (2012) An artificial neural network method for remaining useful life prediction of equipment subject to condition monitoring. J Intell Manuf 23:227–237. https://doi.org/10.1007/s10845-009-0356-9
Freiesleben J, Keim J, Grutsch M (2020) Machine learning and Design of Experiments: Alternative approaches or complementary methodologies for quality improvement? Qual Reliab Eng Int 36:1837–1848. https://doi.org/10.1002/qre.2579
Acknowledgements
The editorial board is kindly asked to consider acknowledging the current research for potential publication in the Silicon journal. The material presented is novel and has not been previously published.
Funding
On behalf of the corresponding author Sudish Mishra, I certify that the current research did not receive funding from any external agency.
Author information
Authors and Affiliations
Contributions
A: Corresponding Author has drafted the whole manuscript B: Second Author has contributed towards design of manuscript C: Third author checked for any grametical error or done the proofreading of whole manuscript.
Corresponding author
Ethics declarations
Ethical Approval
As the representative of the other co-authors, I confirm that we have obtained ethical approval to publish the data included in the manuscript. Additionally, we have appropriately referenced any data utilized in this paper, including figures.
Consent to participate
NA.
Consent of Publication
As the representative of the other co-authors, I confirm that we have obtained permission to publish the current content.
Research Involving Human Participants
The current research involves human participants.
Informed Consent
NA.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, S., Sharma, L. & Chhibber, R. Modelling and Thermophysical Properties of TiO2-SiO2-CaO and SiO2-CaO-MgO Based Electrode Coatings for Offshore Applications. Silicon 15, 7015–7037 (2023). https://doi.org/10.1007/s12633-023-02559-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-023-02559-4