Abstract
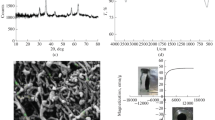
In the work, synthesis and application of the ternary nanocomposite of SiO2/Fe3O4/multi-walled carbon nanotubes (SFCNT) for adsorptive removal of malachite green (MG) from aqueous solutions are reported. A Box-Behnken experimental design with the variables of SFCNT dosage, contact time, pH and ionic strength was used to optimize the effects of the variables on the decolourization process. The results were satisfactorily fitted to a quadratic response surface model with R2=0.9735 and F=36.78 which predicted the optimum conditions of operation (SFCNT dosage of 0.192 g L-1, contact time of 25.1 minutes, pH of 6.26, and 0.03 mol L-1 ionic strength) and the removal efficiency of 98.42±0.18% was achieved. The adsorption data obtained for different MG concentrations showed good agreement with the pseudo-second order kinetic model (R2>0.99). The data were also fitted to different isotherms (Langmuir, Tempkin, Harkins-Jura, Jovanovic, Halsey and Freundlich) and the results of error analyses showed that Freundlich isotherm was the best model to describe the dye-nanocomposite adsorption system.
Similar content being viewed by others
Change history
19 December 2019
After a more careful reconsideration of the article (Fariba Safa<Superscript>*</Superscript>, Yousef Alinezhad, <Emphasis Type="Italic">Silicon</Emphasis>, doi:<ExternalRef><RefSource>https://doi.org/10.1007/s12633-019-00251-0</RefSource><RefTarget Address="10.1007/s12633-019-00251-0" TargetType="DOI"/></ExternalRef>), it was found that the words: ″Deviation of ″ are missing from the last sentence of section 3.7. The issue is fixed via the present Erratum
References
Bhushan B (2010) Springer Handbook of nanotechnology. Springer-Verlag, Berlin Heidelberg
Tasis D, Tagmatarchis N, Bianco A, Prato M (2006) Chemistry of carbon nanotubes. Chem. Rev. 106:1105–1136
Tang WW, Zeng GM, Gong JL, Liu Y, Wang XY, Liu YY, Liu ZF, Chen L, Zhang XR, Tu D (2012) Simultaneous adsorption of atrazine and Cu (II) from wastewater by magnetic multi-walled carbon nanotube. Chem. Eng. J. 211:470–478
Maggini L, Raquez JM, Marega R, Jensen Ahrens J, Pineux F, Meyer F, Dubois P, Bonifazi D (2013) Magnetic poly(vinylpyridine)-coated carbon nanotubes: An efficient supramolecular tool for wastewater purification. ChemSusChem 6:367–373
Speltini A, Merli D, Profumo A (2013) Analytical application of carbon nanotubes, fullerenes and nanodiamonds in nanomaterials-based chromatographic stationary phases: A review. Anal. Chim. Acta 783:1–16
Karimi R, Yousefi F, Ghaedi M, Dashtian K (2016) Back propagation artificial neural network and central composite design modeling of operational parameter impact for sunset yellow and azur (II) adsorption onto MWCNTS and MWCNTS-Pd-NPs: Isotherm and kinetic study. Chemom. Intell. Lab. Syst. 159:127–137
Ferreira GMD, Ferreira GMD, Hespanhol MC, Rezende JP, Pires ACS, Gurgel LVA, Silva LHM (2017) Adsorption of red azo dyes on multi-walled carbon nanotubes and activated carbon: A thermodynamic study. Colloids Surf. A: Physicochem. Eng. Asp. 529:531–540
Zare K, Sadegh H, Shahryari-ghoshekandi R, Maazinejad B, Ali V, Tyagie I, Agarwal S, Gupta VK (2015) Enhanced removal of toxic Congo red dye using multi-walled carbon nanotubes: Kinetic, equilibrium studies and its comparison with other adsorbents. J. Mol. Liq. 212:266–271
Wang JP, Yang HC (2010) Adsorption of phenol and basic dye on carbon nanotubes/carbon fabric composites from aqueous solution. Sep. Sci. Technol. 46:340–348
Xiao DL, Li H, He H, Lin R, Zuo PL (2014) Adsorption performance of carboxylated multi-wall carbon nanotube-Fe3O4 magnetic hybrids for Cu(II) in water. New Carbon Mater. 29:15–25
Luo X, Zhang L (2009) High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon. J. Hazard. Mater. 171:340–347
Li X, Zhu G, Luo Y, Yuan B, Feng Y (2013) Synthesis and applications of functionalized magnetic materials in sample preparation. Trends Anal. Chem. 45:233–247
Alimohammadi V, Sedighi M, Jabbari E (2017) Experimental study on efficient removal of total iron from wastewater using magnetic-modified multi-walled carbon nanotubes. Ecol. Eng. 102:90–97
Sadegh H, Ali GAM, Makhlouf ASH, Chong KF, Alharbi NS, Agarwal S, Gupta VK (2018) MWCNTs-Fe3O4 nanocomposite for Hg(II) high adsorption efficiency. J. Mol. Liq 258:345–353
Suwattanamala A, Bandis N, Tedsree K, Issroc C (2017) Synthesis, characterization and adsorption properties of Fe3O4/MWCNT magnetic nanocomposites. Mater. Today Proc. 4:6567–6575
Kerke Ö, Bayazit ŞS (2014) Magnetite decorated multi-walled carbon nanotubes for removal of toxic dyes from aqueous solutions. J. Nanopart. Res. 16:2431
Fazelirad H, Ranjbar M, Taher MA, Sargazi G (2015) Preparation of magnetic multi-walled carbon nanotubes for an efficient adsorption and spectrophotometric determination of amoxicillin. J. Ind. Eng. Chem. 21:889–892
Chung MH, Chen LM, Wang WH, Lai Y, Yang PF, Lin HP (2014) Effects of mesoporous silica coated multi-wall carbon nanotubes on the mechanical and thermal properties of epoxy nanocomposites. J. Taiwan Inst. Chem. Eng. 45:2813–2819
Sodipo BK, Aziz AA (2013) Sonochemical synthesis of silica coated superpara-magnetic iron oxide nanoparticles. Mater. Sci. Forum 756:74–79
Gawande MB, Monga Y, Zboril R, Sharma RK (2015) Silica-decorated magnetic nanocomposites for catalytic applications. Coord. Chem. Rev. 288:118–143
Kayode SB, Aziz AA (2014) An in-situ functionalization of decanethiol monolayer on thin silica coated superparamagnetic iron oxide nanoparticles synthesized by non-seeded process. Adv. Mater. Res. 1024:300–303
Zhu J, Wei S, Gu H, Rapole SB, Wang Q, Luo Z, Haldolaarachchige N, Young DP, Guo Z (2011) One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal. Environ. Sci. Technol. 46:977–985
Heidari H, Razmi H, Jouyban A (2012) Preparation and characterization of ceramic/ carbon coated Fe3O4 magnetic nanoparticle nanocomposite as a solid-phase microextraction adsorbent. J. Chromatogr. A 1245:1–7
Peng X, Wang Y, Tang X, Liu W (2011) Functionalized magnetic core–shell Fe3O4@SiO2 nanoparticles as selectivity-enhanced chemosensor for Hg(II). Dyes Pigments 91:26–32
Box GEP, Draper NR (1987) Empirical model-building and response surfaces. Wiley, Minnesota
Kleijnen JPC (2015) Response surface methodology, Handbook of simulation optimization. Springer, New York
Bandari F, Safa F, Shariati S (2015) Application of Response Surface Method for Optimization of Adsorptive Removal of Eriochrome Black T Using Magnetic Multi-Wall Carbon Nanotube Nanocomposite. Arab. J. Sci. Eng. 40:3363–3372
Ehyaee M, Safa F, Shariati S (2017) Magnetic Nanocomposite of Multi-walled Carbon Nanotube as Effective Adsorbent for Methyl Violet Removal from Aqueous Solutions: Response Surface Modeling and Kinetic Study. Korean J. Chem. Eng. 34:1051–1061
Tavakoli M, Safa F, Abedinzadeh N (2019) Binary nanocomposite of Fe3O4/MWCNTs for adsorption of Reactive Violet 2: Taguchi design, kinetics and equilibrium isotherms, Fullerenes. Nanotubes and Carbon Nanostructures 27(4):305–316
Gong R, Jin Y, Chenc F, Chenb J, Liu Z (2006) Enhanced malachite green removal from aqueous solution by citric acid modified rice straw. J. Hazard. Mater. 137:865–870
Mitrowska K, Posyniak A (2004) Determination of malachite green and its metabolite, leucomalachite green, in fish muscle by liquid chromatography. Bull. Vet. Inst. Pulawy 48:173–176
Petcharoen K, Sirivat A (2012) Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 177:421–427
Chen J, Zhu X (2015) Ionic liquid coated magnetic core/shell Fe3O4@SiO2 nanoparticles for the separation/analysis of linuron in food samples. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 137:456–462
Box GEP, Hunter JS, Hunter WG (2005) Statistics for experiments2nd edn. Wiley Interscience, New York
German-Heins J, Flury M (2000) Sorption of Brilliant Blue FCF in soils as affected by pH and ionic strength. Geoderma 97:87–101
An Y, Yang L, Hou J, Liu ZY, Peng BH (2014) Synthesis and characterization of carbon nanotubes-treated Ag@TiO2 core–shell nanocomposites with highly enhanced photocatalytic performance. Opt. Mater. 36:1390–1395
Goyanes S, Rubiolo GR, Salazar A, Jimeno A, Corcuera MA, Mondragon I (2007) Carboxylation treatment of multi-walled carbon nanotubes monitored by infrared and ultraviolet spectroscopies and scanning probe microscopy. Diamond Relat. Mater. 16:412–417
Waldron RD (1955) Infrared Spectra of ferrites. Phys. Rev. 99:1727–1735
Yetilmezsoy K, Demirel S, Vanderbei RJ (2009) Response surface modeling of Pb(II) removal from aqueous solution by Pistacia vera L.: Box–Behnken experimental design. J. Hazard. Mater. 171:551–562
Qu S, Huang F, Yu S, Chen G, Kong J (2008) Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J. Hazard. Mater. 160:643–647
Pillay K, Cukrowska EM, Coville NJ (2009) Multi-walled carbon nanotubes as adsorbents for the removal of parts per billion levels of hexavalent chromium from aqueous solution. J. Hazard. Mater. 166:1067–1075
Lagergren S (1898) About the theory of so called adsorption of soluble substances. Ksver Veterskapsakad Handl. 24:1–6
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem. 34:451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 89:31–60
Kannan K, Sundaram MM, Sundaram (2001) Kinetics and mechanism of removal of Methylene Blue by adsorption on various carbons–A comparative study. Dyes Pigm. 51:25–40
Zhang Z, Zhang Z, Fernandez Y, Menendez JA, Niu H, Peng J, Zhang L, Guo S (2010) Adsorption isotherms and kinetics of methylene blue on a low-cost adsorbent recovered from a spent catalyst of vinyl acetate synthesis. Appl. Surf. Sci. 256:2569–2576
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc 40:1361–1403
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 5:212–223
Tempkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochimica USSR 12:327–356
Harkins WD, Jura EJ (1944) The decrease of free surface energy as a basis for the development of equations for adsorption isotherms; and the existance of two condensed phases in films on solids. J. Chem. Phys. 12:112–113
Jovanovic DS (1969) Physical adsorption of gases. Colloid-Z.Z Polym. 235:1214–1225
Halsey G (1948) Physical adsorption on non-uniform surfaces. J. Chem. Phys. 16:931–937
Freundlich HZ (1906) Over the adsorption in solution. J. Phys. Chem. 57:385–471
Silva SM, Sampaio KA, Ceriani R, Verbe R, Stevens C, De Greyt W, Meirelles AJA (2013) Adsorption of carotenes and phosphorus from palm oil onto acid activated bleaching earth: equilibrium, kinetics and thermodynamics. J. Food Eng. 118:341–349
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 156:2–10
Treybal RE (1980) Mass transfer operations3rd edn. McGraw-Hill, New York
Porter JF, McKay G, Choy KH (1999) The prediction of sorption from a binary mixture of acidic dyes using single- and mixed-isotherm variants of the ideal adsorbed solute theory. Chem. Eng. Sci. 54:5863–5885
Syafiuddin A, Salmiati S, Jonbi J, Fulazzaky MA (2018) Application of the kinetic and isotherm models for better understanding of the behaviors of silver nanoparticles adsorption onto different adsorbents. J. Environ. Manag. 218:59–70
Mall ID, Srivastava VC (2005) Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids Surf. A: Physicochem. Eng. Asp. 264:17–28
Uma BS, Sharma YC (2013) Equilibrium and kinetic studies for removal of malachite green from aqueous solution by a low cost activated carbon. J. Ind. Eng. Chem. 19:1099–1105
Ghaedi M, Mosallanejad N (2014) Study of competitive adsorption of malachite green and sunset yellow dyes on cadmium hydroxide nanowires loaded on activated carbon. J. Ind. Eng. Chem. 20:1085–1096
Setareh Derakhshan M, Moradi O (2014) The study of thermodynamics and kinetics methyl orange and malachite green by SWCNTs, SWCNT-COOH and SWCNT-NH2 as adsorbents from aqueous solution. J. Ind. Eng. Chem. 20:3186–3194
Rajabi M, Mirza B, Mahanpoor K, Mirjalili M, Najafi F, Moradi O, Sadegh H, Shahryari-ghoshekandi R, Asif M, Tyagi I, Agarwal S, Gupta VK (2016) Adsorption of malachite green from aqueous solution by carboxylate group functionalized multi-walled carbon nanotubes: Determination of equilibrium and kinetics parameters. J. Ind. Eng. Chem. 34:130–138
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Safa, F., Alinezhad, Y. Ternary nanocomposite of SiO2/Fe3O4/Multi-Walled Carbon Nanotubes for Efficient Adsorption of Malachite Green: Response Surface Modeling, Equilibrium Isotherms and Kinetics. Silicon 12, 1619–1637 (2020). https://doi.org/10.1007/s12633-019-00251-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-019-00251-0