Abstract
Background
Postoperative vomiting (POV) in children is frequent. Dextrose-containing intravenous fluids in the perioperative period have shown improvement of POV in adults. Similar studies have not been done in children.
Aim
The primary purpose was to study the efficacy of intraoperative intravenous dextrose for antiemetic prophylaxis in children undergoing ambulatory surgery.
Methods
A non-inferiority randomized clinical trial of healthy children (three to nine years old) undergoing ambulatory dental surgery was conducted. The control group received dexamethasone (0.15 mg·kg−1 iv) and ondansetron (0.05 mg·kg−1 iv); the intervention group received dexamethasone (0.15 mg·kg−1 iv) and intravenous 5% dextrose in 0.9% normal saline according to a weight-based maintenance rate. The primary outcome was POV in the postanesthetic care unit (PACU) within two hr after surgery. Secondary outcomes included POV within 24 hr from discharge and unplanned hospital admission. A non-inferiority analysis was conducted on the primary outcome using an absolute risk difference of 7.5% as the non-inferiority margin.
Results
Data from 290 patients were analyzed. Demographics and intraoperative anesthetic management were similar between groups. Vomiting in the PACU occurred in 7.6% and 3.5% of the dextrose and ondansetron groups, respectively, with a risk difference of 4.2% (95% confidence interval [CI], -1.0 to 9.5). Given that the upper limit of the 95% CI exceeded our non-inferiority margin, non-inferiority of dextrose compared with ondansetron was not shown.
Conclusion
These results do not support the use of intravenous dextrose as a satisfactory alternative to ondansetron to prevent POV in ambulatory pediatric dental surgery patients.
Trial registration
www.clinicaltrials.gov (NCT 01912807); registered 18 July 2013.
Résumé
Contexte
Les vomissements postopératoires (VPO) sont fréquents chez l’enfant. Il a été démontré qu’en période périopératoire, les solutés intraveineux contenant du dextrose entraînaient une diminution des VPO chez l’adulte, mais des études similaires n’ont pas été réalisées auprès de populations pédiatriques.
Objectif
L’objectif principal était d’évaluer l’efficacité du dextrose intraveineux peropératoire en tant que prophylaxie antiémétique chez les enfants subissant une chirurgie ambulatoire.
Méthode
Une étude clinique randomisée de non-infériorité a été réalisée auprès d’enfants en bonne santé (de trois à neuf ans) devant subir une chirurgie dentaire en ambulatoire. Le groupe témoin a reçu de la dexaméthasone (0,15 mg·kg−1 iv) et de l’ondansétron (0,05 mg·kg−1 iv); le groupe intervention a reçu de la dexaméthasone (0,15 mg·kg−1 iv) et du dextrose intraveineux 5 % dans une solution de normal salin 0,9 % selon une échelle basée sur le poids. Le critère d’évaluation principal était la présence de VPO en salle de réveil au cours des deux heures suivant la chirurgie. Les critères d’évaluation secondaires comprenaient les VPO au cours des 24 h suivant le congé et une admission non planifiée à l’hôpital. L’analyse de non-infériorité a été réalisée pour le critère d’évaluation primaire en se fondant sur une différence de risque absolu de 7,5 % comme marge de non-infériorité.
Résultats
Les données de 290 patients ont été analysées. Les données démographiques et de prise en charge anesthésique peropératoire étaient semblables entre les deux groupes. Des vomissements sont survenus en salle de réveil chez 7,6 % et 3,5 % des groupes dextrose et ondansétron, respectivement, avec une différence de risque de 4,2 % (intervalle de confiance [IC] 95 %, -1,0 à 9,5). Étant donné que la limite supérieure de l’IC 95 % excédait notre marge de non-infériorité, la non-infériorité du dextrose comparativement à l’ondansétron n’a pas été démontrée.
Conclusion
Ces résultats n’appuient pas l’utilisation de dextrose intraveineux en tant qu’alternative à l’ondansétron afin de prévenir les VPO chez les patients pédiatriques de chirurgie dentaire ambulatoire.
Enregistrement de l’étude
www.clinicaltrials.gov(NCT 01912807); enregistrée le 18 juillet 2013.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Postoperative nausea and vomiting (PONV) continues to be a common complication amongst surgical patients, especially children, despite appropriate prophylaxis.1 It has been regarded as the fourth most common indication for unanticipated hospitalization after ambulatory surgery in children.2 Postoperative nausea and vomiting is generally a distressing postoperative morbidity that usually lacks long-term effects, but sequelae can include dehydration, electrolyte abnormalities, suture dehiscence, bleeding, and life-threatening airway compromise.3
In comparison with adults, children have a higher inherent risk of PONV, therefore warranting pediatric specific PONV guidelines.4 As per these guidelines, a multimodal approach to preventing PONV has advocated for using pharmacologic agents of different classes and that act via different mechanisms.1 A novel PONV prophylaxis method studied in adults is the perioperative administration of dextrose-containing intravenous solutions.5 Considering the long safety record of dextrose-containing crystalloid maintenance solutions in children, use of dextrose for PONV prophylaxis warrants study.6,7
In this study, we hypothesized that intraoperative intravenous dextrose was non-inferior to ondansetron for antiemetic prophylaxis in children undergoing ambulatory dental surgery.
Methods
This study was approved by the University of Saskatchewan Biomedical Research Ethics Board (Bio# 13-163). The study protocol was initially registered in July 2013 at clinicaltrials.gov (NCT 01912807).
This study was conducted in an ambulatory surgical centre from December 2013 to August 2014. It was a non-inferiority randomized-controlled trial with parallel assignment of participants. Subjects, caregivers, healthcare providers, and clinical investigators were blinded to group assignment throughout the perioperative period.
Healthy children were recruited for the study that met the following inclusion criteria: age three to nine years, minimal perioperative risk, American Society of Anesthesiology physical status classification I or II, and undergoing ambulatory dental surgery. Children with any underlying pro-emetic disease, personal history of diabetes, positive personal or familial history of postoperative vomiting (POV), or those concurrently taking antiemetic medications were excluded. Written informed parental/legal guardian consent and participant assent were obtained preoperatively.
Randomization consisted of 50 blocks of six patients. Participants were randomized into one of two groups based on antiemetic prophylaxis. The intervention group (144 participants) received dexamethasone (mg dose kg−1 iv; maximum 5 mg) and intravenous 5% dextrose in 0.9% normal saline (D5NS) maintenance fluid. The control group (146 participants) received dexamethasone (0.15 mg·kg−1 iv; maximum 5 mg) and ondansetron (0.05 mg·kg−1 iv; maximum 4 mg) for POV prophylaxis; the treatment given to the control group was similar to that one used in the reference study.8 All doses were based on guideline recommendations for pediatric ambulatory surgery.1 Allocation details were stored in numbered, sealed, and opaque envelopes. Treatment allocation was revealed to the anesthesiologists by opening the envelope prior to surgery.
The research assistant prepared the study drug (ondansetron or normal saline [NS]) in identically appearing clear syringes (study drug “A or B”) and covered the commercial labelling on intravenous solutions (NS vs D5NS). Kits appropriate for both control and intervention groups were made before patient recruitment with the necessary contents for the study, except for dexamethasone, which was given by the anesthesiologists from the anesthesia medications stock.
The intravenous solution was placed in an intravenous infusion pump (Plum® A+ Infusion System, Hospira Inc, Lake Forest, IL, USA) by the researcher and the infusion rate was calculated based on standard weight-based pediatric maintenance rates following the 4:2:1 rule.9 Once intravenous access was established and the patient was intubated, the study solution was connected and infused throughout the operative period. Additional fluid (Ringer’s lactate) was available to the anesthesiologists to administer as per their preference.
There was no standardized protocol for anesthetic induction and maintenance, and all types and doses of anesthetic medications were chosen and administered at the discretion of the anesthesiologist. Intraoperative administration of any other antiemetic medications constituted protocol violation. There were no modifications to the planned dental procedure.
The study drug was administered to the patient by the anesthesiologist at the end of the procedure, when the throat packing was removed, and the intravenous maintenance solution was stopped. Before emergence from anesthesia, the researcher measured and recorded the patient’s blood sugar using a glucometer (AccuCheck aviva®, Roche Diagnostics GmbH, Mannheim, Germany). Ringer’s lactate intravenous fluid, not part of the study protocol, was continued in recovery based on the anesthesiologist’s preference.
All patients were transferred to the postanesthetic care unit (PACU) at the end of the procedure. Discharge from the PACU was based on the Post Anesthetic Discharge Scoring System and institutional guidelines.10 Nursing staff and researchers recorded the presence and incidence of POV in the PACU. Analgesics and antiemetic agents were prescribed by the anesthesiologist during the recovery period and given according to nursing assessment based on institutional guidelines. Researchers telephoned participants 24 hr after discharge to inquire about incidence of emesis and any need to seek medical attention after being discharged from the institution.
Data collection
Researchers were blinded to group assignment. The primary outcome was incidence of emesis within two hours of PACU admission. The timing of POV was described as occurring early (zero to two hours) or late in the recovery period (within 24 hr of facility discharge) for analysis. Secondary outcomes included incidence and number of episodes of emesis occurring within the late recovery period, usage of rescue antiemetic medications in the early and late recovery period, intraoperative blood glucose levels, unplanned hospital admission for POV, delays in discharge from PACU due to POV, and return to hospital/medical assessment because of POV. Intraoperative data including anesthetic medications and doses administered were recorded in the operating room and obtained from the anesthetic record.
Statistical analysis
Statistical analysis was performed with the assistance of the University of Saskatchewan Clinical Research Support Unit using SAS software (v 9.4; SAS Cary, NC, USA).
The sample size was calculated based on the null hypothesis that the early POV rate in the intervention group would be 7.5% more (non-inferiority margin based on clinical judgement and literature review) than the control group, with a reference of early POV rate in the control group of 25%.8,11 Using a power of 80% and a significance of 5%, a sample size of 284 participants was calculated. To account for the usual dropouts and technical problems, a total sample size of 300 subjects was chosen.
We elected to employ a non-inferiority trial design for this clinical trial. This was chosen because non-inferiority trials show that an intervention is “not worse” compared with a standard therapy.12 The decision to pursue a non-inferiority study protocol was influenced by ethical considerations, based on international recommendations by the United States Food and Drug Administration (FDA).13 The FDA suggests including an active control when studying groups at high risk of developing the condition being studied. The magnitude of our non-inferiority margin was guided by the results of a randomized-controlled trial conducted in children with similar demographic characteristics to our study population.8 This study compared the difference in POV rates between children receiving prophylaxis and those receiving dexamethasone alone or in combination with ondansetron and the protective effect of both dexamethasone and ondansetron. This study showed an absolute risk reduction in POV rates of 18% in the group receiving ondansetron.8
Our primary outcome was analyzed using an intention to treat analysis. To test the non-inferiority of the intervention, the upper limit of the two-sided 95% confidence interval (CI) for the proportion of early POV proportion difference between the intervention and control groups had to lie below the predefined 7.5% non-inferiority margin. Outcomes were assessed for normality and analyzed by parametric and nonparametric statistics accordingly. To examine the association between categorical variables, analysis via the Chi squared test (or Fisher’s exact test when more than 20% of cells had expected counts less than five) was used. Normally-distributed variables were reported as mean (standard deviation [SD]). Continuous variables that did not meet normality criteria were reported as median [interquartile range (IQR)]. A two-sample t test was used to compare means between groups for continuous normally distributed variables. Continuous, non-normally distributed variables were compared using Wilcoxon tests when analyzing two samples (majority of the analysis), and Kruskal–Wallis tests when analyzing more than two samples, such as comparing the total amount of intravenous fluids administered (< 200 mL, 200 mL to 450 mL, and > 450 mL) to proportion of vomiting. The level of statistical significance was set at 0.05 (two-tailed).
Results
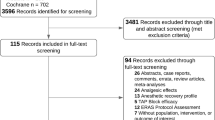
A total of 300 participants were enrolled in this clinical trial. Ten participants were deemed ineligible (nine had first-degree relatives with a history of PONV and one had a personal history of PONV). Data from 290 participants were analyzed including one patient who received an antiemetic outside of the study protocol (intention to treat analysis) as shown in the Figure.

Adapted from the CONSORT 2010 flow diagram, transparent reporting of trials http://www.consort-statement.org.
Clinical trial report based on 2010 CONSORT flow diagram.
Demographic characteristics among groups were similar (Table 1). Despite not having a standardized perioperative anesthetic management procedure implemented as part of the study protocol, the distribution of intraoperative anesthetic medications used between groups was similar with respect to the total intravenous fluid administered (Tables 2 and 3).
Primary and secondary outcomes
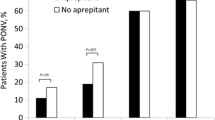
As shown in Table 4, the primary outcome, emesis in the PACU, was not significantly different between groups; early POV occurred in 7.64% of patients in the intervention group and in 3.45% of patients in the control group, with a risk difference of 4.2% (95% CI, -1.0 to 9.5) (Table 4). Because the upper limit (9.5%) exceeded our a priori non-inferiority margin (7.5%), we did not show non-inferiority of dextrose compared with ondansetron.
Analysis of secondary outcomes revealed the use of antiemetic rescue medications was not significantly different between groups (Table 4); 3% of intervention group participants received such therapy while none in the control group did. There was a statistically significant difference in blood glucose levels between groups with the mean levels in the intervention group being 0.8 mmol·L−1 higher than the active control (95% CI, 0.5 to 1.0), as shown in Table 4. Two patients in the intervention group were delayed in discharge from PACU because POV, while no delays occurred in the control group. This difference was not statistically significant.
Discussion
Based on the FDA and CONSORT guidelines for analysis and reporting non-inferiority clinical trials,13,14,15 our results did not support our hypothesis that intravenous dextrose-containing solutions in combination with dexamethasone would be non-inferior to ondansetron in combination with dexamethasone for prevention of POV amongst pediatric day surgery patients. To our knowledge, our study is the first to offer insight into the efficacy of intravenous dextrose on the incidence of POV in children.
Strengths of this study include those associated with a randomized-controlled trial. The randomization appeared to avoid selection bias with similar patient demographic and exposures to associated pro-emetic factors despite not specifying an anesthetic protocol. Another strength of the study was the sample size, which was larger than those in similar studies performed in adults and children, further decreasing potential bias.
Our study had limitations. First, we did not include a true placebo arm. We felt it unethical to withhold PONV prophylaxis as per recognized guidelines to a study population with a high inherent risk of developing POV.2 Another limitation was our inability to record or analyze the incidence of postoperative nausea in our study because there is no validated pediatric nausea scoring system. Severity of nausea scoring systems have been validated in pediatric cancer patients undergoing chemotherapy over seven years of age, but a descriptive tool was lacking for most of our study population.16 Thus, the common postoperative complication of nausea without vomiting may have been present and could have been significantly different between both groups. Additionally, this study was underpowered to make meaningful conclusions. Further study in this field may consider using our results to guide more appropriate sample size estimations. Finally, the lack of a standardized anesthetic protocol could account for an unknown possible bias in the study, although randomization was used to minimize this possibility and caregivers were blinded to treatment allocation.
The association between perioperative sugar administration in adult patients and a reduction in PONV is not a novel idea. Previous investigation in adult gynecologic patients has shown that postoperative dextrose administration decreases antiemetic rescue administration and PACU length of stay.5 Another study in adult gynecologic patients compared the effects of crystalloid with and without 50% dextrose administered early in the surgical procedure.17 The results showed increased PONV, PACU narcotic use, and thirst in the intervention group. Although studies examining the effects of oral dextrose loading preoperatively have shown less POV and improved recovery,18 administering oral dextrose solutions prior to general anesthetic may increase the risk of aspiration.
Previous authors have questioned the safety of dextrose-containing intravenous solutions administered to children.19 A theoretical concern of using the solution in our protocol is its hypertonicity (586 mmol·L−1) and the risks of altering circulation volume. Previous studies have shown that similar dextrose solutions only cause transient changes in circulating volume because of rapid dextrose metabolism. Another concern of administering intravenous dextrose is hyperglycemia and its associated risks, including inappropriate diuresis, electrolyte disturbances, and wound infection.19,20 In children, glucose-containing solutions are also used to prevent possible hypoglycemia during surgery. For this purpose, when using intravenous 5% dextrose-containing solutions, it has been recommended to use infusion rates of 3-4 mg·kg−1·min−1 to avoid hyperglycemia.19 Our study protocol used a dextrose infusion rate of 2–3.5 mg·kg−1·min−1, depending on the patient’s weight and the length of the procedure. Further, the mean glucose level in the intervention group was less than that defined as hyperglycemia in terms of diabetes.21 Despite the difference in blood glucose levels between groups, this difference was likely not clinically significant and did not warrant intervention at any time.19
Our findings failed to support the use of dextrose in combination with dexamethasone when compared with ondansetron in combination with dexamethasone for PONV prophylaxis in children. Future trials are needed to define the role of intravenous sugar in reducing POV.
References
Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2014; 118: 85-113.
Kovac AL. Management of postoperative nausea and vomiting in children. Paediatr Drugs 2007; 9: 47-69.
Scuderi PE, Conlay LA. Postoperative nausea and vomiting and outcome. Int Anesthesiol Clin 2003; 41: 165-74.
Eberhart L, Geldner G, Kranke P, et al. The development and validation of a risk score to predict the probability of postoperative vomiting in pediatric patients. Anesth Analg 2004; 99: 1630-7.
Dabu-Bondoc S, Vadivelu N, Shimono C, et al. Intravenous dextrose administration reduces postoperative antiemetic rescue treatment requirements and postanesthesia care unit length of stay. Anesth Analg 2013; 117: 591-6.
Dubois MC, Gouyet L, Murat I, Saint-Maurice C. Lactated Ringer with 1% dextrose: an appropriate solution for peri-operative fluid therapy in children. Paediatr Anaesth. DOI: https://doi.org/10.1111/j.1460-9592.1992.tb00183.x
Levy JA, Bachur RG. Intravenous dextrose during outpatient rehydration in pediatric gastroenteritis. Acad Emerg Med 2007; 14: 324-30.
Splinter WM. Prevention of vomiting after strabismus surgery in children: dexamethasone alone versus dexamethasone plus low-dose ondansetron. Paediatr Anaesth 2001; 11: 591-5.
Meyers RS. Pediatric fluid and electrolyte therapy. J Paediatr Pharmacol Ther 2009; 14: 204-11.
Ead H. From Aldrete to PADSS: reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs 2006; 21: 259-67.
Shen Y, Chen C, Wu C, Cherng Y, Tam K. Dexamethasone, ondansetron, and their combination and postoperative nausea and vomiting in children undergoing strabismus surgery: a meta-analysis of randomized controlled trials. Paediatr Anaesth 2014; 24: 490-8.
Christensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol 2007; 46: 947-54.
US Department of Health and Human Services Food and Drug Administration. Non-inferiority clinical trials to establish effectiveness – guidance for industry, 2016. Available from URL: http://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf (accessed May 2020).
Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials 2011. DOI: https://doi.org/10.1186/1745-6215-12-106.
Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012; 308: 2594-604.
Baxter AL, Watcha MF, Baxter WV, Leong T, Wyatt MM. Development and validation of a pictorial nausea rating scale for children. Pediatrics 2011; 127: e1542-9.
McCaul C, Moran C, O’Cronin D, et al. Intravenous fluid loading with or without supplementary dextrose does not prevent nausea, vomiting and pain after laparoscopy. Can J Anesth 2003; 50: 440-4.
Hausel J, Nygren J, Thorell A, Lagerkranser M, Ljungqvist O. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy. Br J Surg 2005; 92: 415-21.
Leelanukrom R, Cunliffe M. Intraoperative fluid and glucose management in children. Paediatr Anaesth 2000; 10: 353-9.
Anderson DJ, Podgorny K, Berríos-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35: 605-27.
Adamkin DH. Metabolic screening and postnatal glucose homeostasis in the newborn. Pediatr Clin North Am. 2015; 62: 385-409.
Author contributions
Andrea Vasquez-Camargo, Jonathan Gamble, Kelly A. Fedoruk, and Grant G. Miller contributed to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data and drafting the article. Hyun J. June Lim and Prosanta K. Mondal contributed to the analysis and interpretation of data. Juan Martinez contributed to the acquisition of data.
Disclosures
None.
Funding statement
Funding for this study was received from the Department of Surgery, University of Saskatchewan.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Associate Editor, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vasquez-Camargo, A., Gamble, J., Fedoruk, K.A. et al. Intravenous dextrose versus ondansetron for prevention of postoperative vomiting in children: a randomized non-inferiority trial. Can J Anesth/J Can Anesth 67, 1333–1340 (2020). https://doi.org/10.1007/s12630-020-01757-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01757-7