Abstract
Purpose
Vasoplegia is a clinical syndrome marked by severe arteriolar vasodilatation, hypotension, and low systemic vascular resistance refractory to multiple vasopressor treatment. We report our experience with hydroxocobalamin (B12) infusion as a potential rescue adjunct for refractory vasoplegia during cardiopulmonary bypass (CPB).
Methods
We performed a retrospective chart review of 33 patients undergoing cardiac surgery between 1 January 2013 and 31 December 2015, who were given intravenous B12 for refractory hypotension during, or immediately following, CPB. We assessed mean arterial pressure (MAP) responses using semi-parametric group-based models (trajectory analysis). Vasopressor use was evaluated by norepinephrine-equivalent rates calculated five minutes prior, and up to 60 min following, B12 administration.
Results
Patients were mostly male (82%), had a mean (SD) age of 53 (13) yr, and median (IQR) EuroSCORE mortality index of 9 [4-40]. Four patterns of MAP responses to B12 were identified. In Group 1 (“poor responders”) nine of 33 patients (27%) had the highest median [IQR] mortality risk (EuroSCORE 40 [4-52]), lowest mean pre-B12 MAP (50 mmHg), and minimal hemodynamic response in spite of continued vasopressor support. In contrast, Group 2 “responders” (8/33, 24%) showed a brisk MAP response (> 15 mmHg) to B12, sustained for > 60 min post-infusion, with 50% vasopressor reduction. Groups 3 and 4 had the lowest median mortality risk (EuroSCORE 8) and highest pre-B12 MAP (72 mmHg). Although Group 3 patients (“sustainers”; 9/33, 27%) showed a sustained MAP improvement, those in Group 4 (“rebounders”; 7/33, 21%) were characterized by hypertensive overshoot followed by a decrease in MAP.
Conclusion
These data indicate considerable heterogeneity in patient response to B12, potentially dependent on both patient preoperative condition and non-standardized time of administration. B12 may provide a useful alternative therapy for refractory hypotension and vasoplegia, but controlled clinical trials to assess efficacy are needed.
Résumé
Objectif
La vasoplégie est un syndrome clinique caractérisé par une vasodilatation artériolaire importante, une hypotension et une résistance vasculaire systémique basse réfractaire au traitement avec plusieurs vasopresseurs. Nous rapportons ici notre expérience concernant une perfusion d’hydroxocobalamine (B12) en tant qu’adjuvant de sauvetage potentiel pour traiter la vasoplégie pendant la circulation extracorporelle (CEC).
Méthode
Nous avons réalisé une analyse rétrospective des dossiers de 33 patients ayant subi une chirurgie cardiaque entre le 1er janvier 2013 et le 31 décembre 2015, et qui ont reçu de la vitamine B12 en intraveineuse pour traiter une hypotension réfractaire pendant ou immédiatement après la CEC. Nous avons évalué les réponses de la tension artérielle moyenne (TAM) à l’aide de modèles semi-paramétriques basés sur le groupe (analyse de trajectoire). L’utilisation de vasopresseurs a été évaluée en taux équivalents à la norépinéphrine et calculée de cinq minutes avant à 60 min après l’administration de B12.
Résultats
Les patients étaient principalement des hommes (82 %), d’un âge moyen (ÉT) de 53 (13) ans, et présentaient un indice de mortalité EuroSCORE médian (ÉIQ) de 9 [4-40]. Quatre types de réponses de la TAM à la B12 ont été identifiés. Dans le groupe 1 (« faible réponse »), neuf des 33 patients (27 %) ont affiché le risque de mortalité médian [ÉIQ] le plus élevé (EuroSCORE 40 [4-52]), la TAM moyenne la plus basse pré-B12 (50 mmHg) et une réponse hémodynamique minimale malgré un traitement continu de vasopresseurs. En revanche, chez les « répondants » du groupe 2 (8/33, 24 %), la réponse de la TAM à la B12 a été rapide (> 15 mmHg), soutenue pendant > 60 min après la perfusion, et l’administration de vasopresseurs a pu être réduite de 50 %. Les groupes 3 et 4 ont manifesté le risque de mortalité médian le plus faible (EuroSCORE 8) et la TAM pré-B12 la plus élevée (72 mmHg). Bien que chez les patients du groupe 3 (« réponse soutenue »; 9/33, 27 %) l’amélioration de la TAM se soit maintenue, les patients du groupe 4 (« réponse rebond »; 7/33, 21 %) étaient caractérisés par une réponse hypertensive suivi d’une diminution de la TAM.
Conclusion
Ces données indiquent une importante hétérogénéité de la réponse des patients à la vitamine B12, laquelle pourrait potentiellement dépendre tant de la condition préopératoire du patient que du moment non standardisé de l’administration de l’agent. La vitamine B12 pourrait constituer un traitement alternatif utile pour l’hypotension réfractaire et la vasoplégie, mais des essais cliniques contrôlés sont nécessaires pour évaluer son efficacité.
Similar content being viewed by others
Vasoplegia is a clinical syndrome marked by severe arteriolar vasodilatation, hypotension, and low systemic vascular resistance (SVR) refractory to multiple catecholamine or other vasopressor interventions. This syndrome occurs in 5-20% of cardiopulmonary bypass (CPB) cases and can result in mortality rates of 25-50%. However, the optimal choice of interventional agent is subject to debate,1 and the evidence for efficacy of any one drug is generally low to moderate.2
Overproduction of nitric oxide (NO) contributes to both systemic inflammatory responses and hypo-responsiveness to vasopressors.3,4,5,6 Consequently, recent vasoplegia rescue therapies have incorporated the NO synthase inhibitor methylene blue (MB).5,7,8 Data from several small trials suggest MB may reduce vasoplegia duration and vasopressor use, and ultimately morbidity and mortality.4,9 However, MB may precipitate serious adverse events (such as cardiac dysrhythmias, methemoglobinemia, and serotonin toxicity) and interferes with colorimetric laboratory assays10 and tissue oximetry.6,11 More recently, a number of cases have been described where hydroxocobalamin (vitamin B12) was administered for refractory vasoplegia syndrome.12,13,14,15,16 B12 is an NO scavenger commonly used in the treatment of acute cyanide poisoning; administration is associated with clinically significant increases in blood pressure.12,13,14,15,16
The aim of this present report was to describe the results from a retrospective chart review of patients from a single institution undergoing cardiac surgery with CPB, where B12 was used to reverse perioperative or post-CPB hypotension. We describe patterns of B12 use, patient hemodynamic responses, and clinical characteristics in this case series.
Methods
This study was a single-centre retrospective review of patients undergoing CPB who received B12 to treat vasoplegia. The study protocol was approved by the Institutional Review Board (IRB) of the Virginia Commonwealth University Health System (VCUHS), which waived the need for patient consent (IRB HM20006336 CR1). We followed PROCESS (Preferred Reporting Of CasE Series in Surgery)17 guidelines in conducting and reporting this study.
Adult (≥ 18 yr) cardiac surgery patients having an emergency pharmacy order for IV hydroxocobalamin between 1 January 2013 and 31 December 2015 were identified from the VCUHS pharmacy database. Electronic records for these patients were manually reviewed by a single investigator (P.S.) to confirm use of B12 and to identify and eliminate duplicate or erroneously captured records.
Anesthetic induction and maintenance were at the discretion of the attending anesthesiologist and team. Cardiopulmonary bypass management included the use of a standard centrifugal pump, solid open shell reservoir, and cardiotomy suction. Bypass was conducted under moderate (approx. 32°C) systemic hypothermia. Patients were anticoagulated with porcine heparin (300 IU·kg−1 as a pre-CPB loading dose). Non-pulsatile pump flow was maintained at 2.5 L·min−1·m−2, with a target perfusion pressure of 60-80 mmHg. During surgery, blood products and fluids were administered at the discretion of the cardiac team using an established algorithm based on thromboelastography (TEG™), platelet count, fibrinogen, and prothrombin/international normalized ratio. After weaning from CPB, heparinization was reversed using a 1:1 ratio of protamine to the initial heparin dose administered. Mean arterial pressure (MAP) was measured continuously during surgery using a radial or femoral arterial catheter. If two invasive blood pressures were available, we used the higher measurement for analysis. Measurement of cardiac output via placement of pulmonary arterial catheters was not routine clinical practice at this institution.
Emergency off-label use of MB and/or B12 (rescue therapy) was indicated when the attending surgeon and anesthesiologist agreed that there was significant potential for ongoing patient hemodynamic instability to contribute to vasodilatory shock and was sufficiently life-threatening to require immediate intervention. Rescue therapy was initiated if the patient was unable to sustain an MAP > 65 mmHg despite 0.1 µg·kg−1·min−1 norepinephrine, 0.04 unit·min−1 vasopressin, and 0.1 µg·kg−1·min−1 epinephrine. These patients received MB (10 mg·mL−1; 1-2 mg·kg−1) and/or reconstituted hydroxocobalamin (B12; 5 g, 20 mg·mL−1; Cyanokit™; Meridian/Pfizer, Sarasota, FL, USA), administered as an intravenous bolus over 15 min.
Patient demographic, clinical, and laboratory data were obtained from the Society of Thoracic Surgeons (STS) database (Table 1). Intraoperative MAP, vasopressor use, rescue adjuvant drug (MB, B12) use, and time of administration were obtained for patients prior to January 2015 (n = 8) on the Innovian Anesthesia information platform (Dräger Inc; Telford, PA, USA) and after that date (n = 25) on Surginet Anesthesia (Cerner Corporation; Kansas, MO, USA). Vasopressor doses at four standardized times (five minutes before B12 administration; at B12 administration; and 30 and 60 min after B12 administration) were extracted from patient run charts by a single investigator (P.S.). Study data were collated and managed using REDCap electronic data capture tools, hosted at VCUHS.18 Data for each subject were stored on individual spreadsheets and screened for time-stamp and transcription errors.
Statistical analysis
Summary data are reported as mean (standard deviation [SD]) or median (interquartile range [IQR]). Descriptive statistics were calculated in SAS JMP Pro 12.2.0 (SAS Institute; Cary, NC, USA). We used semiparametric group-based mixture models to identify patient clusters of MAP response following B12 administration.19,20 Models were fitted to MAP measurements sampled at five-minute intervals from the time of B12 infusion until 110 min post-infusion. Mean trajectory shape for each group was estimated by polynomial regression using proc traj 19,20 (SAS v.9.4; SAS Institute, Cary, NC, USA). Initially, separate models were built with one through four sub-group trajectories; the best-fit polynomial was then determined for each trajectory by repeated analytic runs until the best combination (constant, linear, quadratic, or cubic) resulted in minimization of the Bayesian information criterion score and a significant P value for each trajectory component.21 Vasopressor dosages were converted to norepinephrine-equivalent units (µg·kg−1·min−1), assuming dose equivalence of 1 µg norepinephrine to 1 µg epinephrine, 0.007 µg dopamine, and 2.5 U vasopressin, respectively.22,23
Results
Of the 117 patient records originally identified and screened, 34 met inclusion criteria in being non-duplicated records of adult cardiac surgery patients undergoing CPB and receiving a single bolus of MB and/or B12. The remaining 83 records were duplicates (n = 3), or referred to non-cardiac surgical patients (n = 19), burn/smoke inhalation (n = 22) patients, or patients who were not recorded as receiving B12 (n = 39). The record for one additional patient was excluded because MAP data were unavailable for the relevant times before and after B12 administration. There were therefore 33 patient records available for analysis.
The mean (SD) patient age was 53 (13) yr, with mean (SD) body mass of 74 (14) kg; 28 of 33 (82%) patients were male. Patient preoperative conditions and risk factors24 are given in Table 1. The median [IQR] EuroSCORE mortality index was 9 [4-40]. Eight of 33 patients (24%) were receiving two or more preoperative medications (ACE inhibitors, beta-blockers, calcium channel blockers, or inotropes) at the time of surgery. Fifteen subjects (45%) had moderate to poor renal function (with two dialysis-dependent subjects). Twenty patients (61%) had poor to severe left ventricular dysfunction (ejection fraction [EF] ≤ 30%), and 20 patients (61%) had moderate to severe pulmonary hypertension.
Intraoperative descriptors and surgical procedures are shown in Table 2. Three patients (9%) experienced cardiac arrest during surgery, but were successfully resuscitated. All patients survived to intensive care unit (ICU) transfer; 30-day mortality was four of 33 patients (12%).
Twenty patients (61%) received B12 during CPB at a median [IQR] time of 76 [28-138] min after the start of CPB, and 13 patients received it at a median [IQR] time of 84 [33-113] min post-CPB. Methylene blue was given to 15 patients (45%), two patients approximately 70 min before CPB, ten patients approximately 100 [35-157] min after CPB start, and three patients approximately 53 min post-bypass. Twelve patients (36%) received MB prior to B12 administration and three patients (9%) after B12 administration (Table 3).
The MAP response following B12 administration was described by a four-group trajectory model, with Groups 1 and 3 described by linear trajectories and Groups 2 and 4 by cubic trajectories (Table 4). Visual inspection (Fig. 1) suggested that these groups could be categorized as “poor responders” (Group 1), “responders” (Group 2), “sustainers” (Group 3), and “rebounders” (Group 4). Groups 1 and 2 (Fig. 1A) consisted primarily (14/17) of patients administered B12 during bypass and with median [IQR] MAP prior to B12 of 55 [48-59] mmHg. Group 1 patients (9/33; 27%) showed little or no immediate response to B12 although MAP continued to increase slowly (0.1 mmHg·min−1) over the next two hours. Three patients received MB before, and one patient 72 min after, B12 administration. Group 2 patients (8/33; 24%) were characterized by a brisk response to B12 within 20 min of administration, followed by sustained maintenance of MAP > 65 mmHg. Three patients received MB before, and one patient over four hours after, B12 administration. Groups 3 and 4 (Fig 1B) consisted mainly of subjects administered B12 after bypass (10/16 subjects) and with MAP at the time of B12 administration above the 65 mmHg threshold (median [IQR] MAP = 71[64-80] mmHg). Group 3 (9/33; 27%) showed a small constant increase in MAP over the duration of the post-B12 monitoring period, resulting in a total increase of approximately 10 mmHg over 110 min. Three patients received MB before B12. In contrast, Group 4 subjects (i.e., “rebounders”; 7/33; 21%) showed an apparent hypertensive overshoot, peaking at MAP > 100 mmHg at approximately 30 min post-B12 infusion, followed by a decline in MAP to approximately 65 mmHg over the next hour. Three patients received MB before B12. Two of these seven patients received further vasopressor infusions, and one patient received MB 80 min after B12 administration.
Four-group trajectory plot of observed and predicted average mean arterial pressure (MAP) of 33 patients administered B12. Predicted values are indicated by heavy dotted lines; 95% confidence intervals by light dotted lines. A) Pre-B12 MAP < 65 mmHg (dashed line). Group 1 (open circles): poor responders; Group 2 (closed circles): responders. B) Pre-B12 MAP > 65 mmHg. Group 3 (open circles): sustained response; Group 4 (closed circles): hypertensive overshoot followed by rebound hypotension
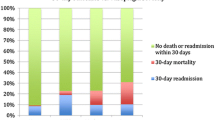
Patient characteristics stratified by MAP response are shown in Table 5. Compared to other groups, Group 1 “non-responders” were sick patients, with a high median [IQR] EuroSCORE mortality index of 40 [4-48]. Compared to other groups, they were characterized by relatively longer surgery times, time on bypass, duration of mechanical ventilation, and length of stay. Two patients in this group subsequently died. Group 2 “responders”, although critically ill (preoperative EF < 30%; median [IQR] EuroSCORE 31 [4-39]), had relatively shorter ventilation duration and length of ICU stay. Two patients in this group subsequently died. Groups 3 and 4 (Fig 1B) had relatively low median [IQR] estimated risk of mortality (EuroSCORE 7 [4-14]), relatively short time on mechanical ventilation, and relatively short ICU stays (Table 5). There were no deaths at 30 days in these groups.
Group 1 patients showed little change in norepinephrine-equivalent vasopressor use over the next hour subsequent to B12 administration. The brisk MAP response and recovery of Group 2 (“responders”) was associated with an approximate 50% reduction of vasopressors by one hour post-B12 infusion. Patients in Group 4 (“rebounders”) also showed an approximate three-fold reduction in vasopressor dosages; Group 3 “sustainers” showed little or no reduction in vasopressor dosages (Fig. 2).
Discussion
In this case series, B12 appeared to benefit a small subset of patients with high preoperative risk of mortality and low perioperative MAP refractory to multiple vasopressors and fluid support. In these patients, MAP increased to > 65 mmHg within 15-30 min and was sustained for the duration of the monitoring period (> 60 min post-B12 administration). In contrast, other patients exhibited apparent B12 hypersensitivity, characterized by MAP overshoot and rebound hypotension, while the most critically ill group of patients showed little or no response. This patient heterogeneity in patient response following B12 administration may reflect the multifactorial etiology of vasoplegia,1 but might also be determined by differences in individual clinician discretion in determining optimal target MAP requirements24 and when to initiate rescue therapy.
Optimal dosing of B12 for vasodilatory shock is unknown. Case studies reported bolus doses of 5 g over 10 to 15 min,12,15,25 as recommended per manufacturer for cyanide toxicity. Case studies of three liver transplant patients reported repeated doses of 125-250 mg supplemented by 250-500 mg·hr−1 infusions.14,16 Higher than recommended dosages of MB have been associated with splanchnic vasospasm; it is unknown if this is a potential problem with B12 administration. Abrupt and prolonged increases in MAP induced by norepinephrine and NO inhibitors in patients with septic shock have been associated with increased mortality24; over the longer term, blood pressure overshoot resulting from excessive vasoconstriction may result in ischemic injury and increased incidence of acute kidney injury and multiple organ failure.26 Optimal timing of B12 administration is also unknown. In septic shock, delayed initiation of fluid and pressor therapies contributes to poor patient outcomes.27 When refractory vasodilatory shock is prolonged, mortality risk is increased, and chances of any therapy resulting in clinical improvement are much lower. In general, both MB and B12 are administered as treatments of last resort; however, B12 has been administered during CPB,12,14,15 and up to 48 hr post-surgery.13
Side effects of B12 administration include chromaturia and interference with colorimetric laboratory tests. Although chromaturia has been described as ‘benign and self-limited’,25 B12 has the potential to disrupt hemodialysis.28 Other than anecdotal reports,25 there are few data on effects of B12 on oximetry devices.
There are some limitations to this case series. Because this was an observational case series, there were no controls. We could not always clearly differentiate whether refractory hypotension was due to low vascular resistance (i.e., vasoplegia) rather than low cardiac output or a central-peripheral blood pressure gradient. Although peripheral and central MAP measurements are considered interchangeable, the relatively wide limits of agreement (16 mmHg) between methods29 may also account in part for differences in MAP. Clinician identification of administration indications was not standardized, thus contributing to patient heterogeneity in response. We expressed vasopressor doses in norepinephrine-equivalent units for consistency with previous literature on vasoplegia.23 However, we acknowledge that this method masks differences in pharmacodynamics and mode of action of the different vasopressor agents. Furthermore, charting of vasopressor use was not uniform across data logging systems, and bolus dosages were frequently omitted from case records, likely underestimating total vasopressor doses. Finally, MAP ‘groups’ identified for these patients result from statistical trajectory analysis modelling with small sample sizes and should be regarded only as heuristic.
Although vitamin B12 administration may provide a useful alternative therapy for refractory hypotension and vasoplegia, randomized controlled clinical trials are needed to assess efficacy, protocols for routine use, and appropriate dosing.30
References
Egi M, Bellomo R, Langenberg C, et al. Selecting a vasopressor drug for vasoplegic shock after adult cardiac surgery: a systematic literature review. Ann Thorac Surg 2007; 83: 715-23.
Gamper G, Havel C, Arrich J, et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev 2016. https://doi.org/10.1002/14651858.CD003709.pub4.
Levin MA, Lin HM, Castillo JG, et al. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegia syndrome. Circulation 2009; 120: 1664-71.
Leyh RG, Kofidis T, Strüber M, et al. Methylene blue: the drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg 2003; 125: 1426-31.
Fischer GW, Levin MA. Vasoplegia during cardiac surgery: current concepts and management. Semin Thorac Cardiovasc Surg 2010; 22: 140-4.
Mittnacht AJ, Fischer GW, Reich DL. Methylene blue administration is associated with decreased cerebral oximetry values. Anesth Analg 2007; 105: 549-50.
Levin RL, Degrange MA, Bruno GF, et al. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg 2004; 77: 496-9.
Maslow AD, Stearns G, Butala P, Schwartz CS, Gough J, Singh AK. The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth Analg 2006; 103: 2-8.
Özal E, Kuralay E, Yildirim V, et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg 2005; 79: 1615-9.
Liu H, Yu L, Tang L, Green MS. Vasoplegic syndrome: an update on perioperative considerations. J Clin Anesth 2017; 40: 63-71.
Jubran A. Pulse oximetry. Crit Care 1999; 3: R11-7.
Roderique JD, Van Dyck K, Holman B, Tang D, Chui B, Spiess BD. The use of high-dose hydroxocobalamin for vasoplegic syndrome. Ann Thorac Surg 2014; 97: 1785-6.
Klemm S, Glienke C. Evaluation of hydroxocobalamin in vasoplegia in cardiac surgery. Crit Care Med 2016. https://doi.org/10.1097/01.ccm.0000508898.30711.31.
Woehlck HJ, Boettcher BT, Lauer KK, et al. Hydroxocobalamin for vasoplegic syndrome in liver transplantation: restoration of blood pressure without vasospasm. A A Case Rep 2016; 7: 247-50.
Burnes ML, Boettcher BT, Woehlck HJ, Zundel MT, Igbal Z, Pagel PS. Hydroxocobalamin as a rescue treatment for refractory vasoplegic syndrome after prolonged cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2017; 31: 1012-4.
Boettcher BT, Woehlck HJ, Reck SE, et al. Treatment of vasoplegic syndrome with intravenous hydroxocobalamin during liver transplantation. J Cardiothorac Vasc Anesth 2016; 31: 1381-4.
Agha RA, Fowler AJ, Rajmohan S, Barai I. Orgill DP; PROCESS Group. Preferred reporting of case series in surgery: the PROCESS guidelines. Int J Surg 2016; 36: 319-23.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377-81.
Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001; 29: 374-93.
Jones BL, Nagin DS. Advances in group-based trajectory modeling and a SAS procedure for estimating them. Ann Am Acad Pol Soc Sci 2007; 35: 542-71.
Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: a tutorial. Tutor Quant Methods Psychol 2009; 5: 11-24.
Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358: 877-87.
Brown SM, Lanspa MJ, Jones JP, et al. Survival after shock requiring high-dose vasopressor therapy. Chest 2013; 143: 664-71.
Mekontso-Dessap A, Houël R, Soustelle C, Kirsch M, Thébert D, Loisance DY. Risk factors for post-cardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg 2001; 71: 1428-32.
Warner MA, Mauermann WJ, Armour S, Barbara DW. Red urinary discolouration following hydroxocobalamin treatment for vasoplegic syndrome. Can J Anesth 2017; 64: 673-4.
Leone M, Asfar P, Radermacher P, Vincent JL, Martin C. Optimizing mean arterial pressure in septic shock: a critical reappraisal of the literature. Crit Care 2015; 19: 101.
Parrillo JE. Septic shock - vasopressin, norepinephrine, and urgency. N Engl J Med 2008; 358: 954-6.
Stellpflug SJ, Gardner RL, Leroy JM, Ellsworth H, Zwank MD. Hydroxocobalamin hinders hemodialysis. Am J Kidney Dis 2013; 62: 395.
Mignini MA, Piacentini E, Dubin A. Peripheral arterial blood pressure monitoring adequately tracks central arterial blood pressure in critically ill patients: an observational study. Crit Care 2006; 10: R43.
Shanmugam G. Vasoplegic syndrome - the role of methylene blue. Eur J Cardiothorac Surg 2005; 28: 705-10.
Acknowledgements
We thank P. Taylor and K. Francis (Pharmacy), M. Haines, M. Copland, and S. Bruffy for assistance with record extraction. We thanks the reviewers for thoughtful and constructive comments that greatly improved the manuscript.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Pranav R. Shah contributed substantially to study conception and design, data collection and interpretation, and drafting the article and approved the final version of the manuscript. Penny S. Reynolds contributed substantially to data archiving, analysis and interpretation, wrote the manuscript, and approved the final version of the manuscript. Nirvik Pal, Daniel Tang, Harry McCarthy, and Bruce D. Spiess contributed to study design, data collection and interpretation, contributed to manuscript drafts, and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, P.R., Reynolds, P.S., Pal, N. et al. Hydroxocobalamin for the treatment of cardiac surgery-associated vasoplegia: a case series. Can J Anesth/J Can Anesth 65, 560–568 (2018). https://doi.org/10.1007/s12630-017-1029-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-1029-3