Abstract
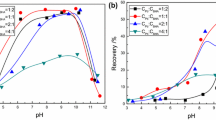
In general, malachite is recovered via sulfidization–xanthate flotation, although many unsatisfactory flotation indexes are frequently obtained as a result of the presence of associated calcite. This phenomenon occurs because the dissolved components of malachite and calcite affect the flotation behavior of both minerals. In this study, the effect of the dissolved components derived from malachite and calcite on the flotation behavior and surface characteristics of both minerals was investigated. Flotation tests indicated that malachite recovery decreased when the calcite supernatant was introduced, while the presence of the malachite supernatant increased the recovery of calcite. Dissolution and adsorption tests, along with zeta potential measurements, X-ray photoelectron spectroscopy, Fourier transform infrared spectrometry, and time-of-flight secondary ion mass spectrometry demonstrated that the Ca species in the calcite supernatant were adsorbed on the malachite surface, which hindered the interaction of Na2S with malachite, thereby resulting in the insufficient adsorption of sodium isoamyl xanthate (NaIX) on the surface of malachite. By contrast, the Cu species in the malachite supernatant were adsorbed on the calcite surface, and they provided active sites for the subsequent adsorption of Na2S and NaIX.
Similar content being viewed by others
References
H. Wang, S. Wen, G. Han, and Q. Feng, Adsorption characteristics of Pb(II) species on the sulfidized malachite surface and its response to flotation, Sep. Purif. Technol., 264(2021), art. No. 118440.
H. Wang, S. Wen, G. Han, and Q. Feng, Modification of malachite surfaces with lead ions and its contribution to the sulfidization flotation, Appl. Surf. Sci., 550(2021), art. No. 149350.
X. Wang, W. Liu, F. Jiao, W. Qin, and C. Yang, New insights into the mechanism of selective flotation of copper and copper-tin alloy, Sep. Purif. Technol., 253(2020), art. No. 117497.
Z. Yin, W. Sun, Y. Hu, C. Zhang, Q. Guan, and K. Wu, Evaluation of the possibility of copper recovery from tailings by flotation through bench-scale, commissioning, and industrial tests, J. Clean. Prod, 171(2018), p. 1039.
J. Li, H. Lu, S. Liu, and Z. Xu, Optimizing the operating parameters of corona electrostatic separation for recycling waste scraped printed circuit boards by computer simulation of electric field, J. Hazard. Mater., 153(2008), No. 1–2, p. 269.
W.Z. Yin, Q.Y. Sun, D. Li, Y. Tang, Y.F. Fu, and J. Yao, Mechanism and application on sulphidizing flotation of copper oxide with combined collectors, Trans. Nonferrous Met. Soc. China, 29(2019), No. 1, p. 178.
X. Chen, Y. Peng, and D. Bradshaw, The separation of chalcopyrite and chalcocite from pyrite in cleaner flotation after regrinding, Miner. Eng., 58(2014), p. 64.
F. Li, X. Zhou, G. Zhao, and R. Lin, A novel decyl-salicyl hydroxamic acid flotation collector: Its synthesis and flotation separation of malachite against quartz, Powder Technol., 374(2020), p. 522.
Q. Zhang, S.M. Wen, Q.C. Feng, and H. Wang, Enhanced sulfidization of azurite surfaces by ammonium phosphate and its effect on flotation, Int. J. Miner. Metall. Mater., 29(2022), No. 6, p. 1150.
G. Han, S. Wen, H. Wang, and Q. Feng, Surface sulfidization mechanism of cuprite and its response to xanthate adsorption and flotation performance, Miner. Eng., 169(2021), art. No. 106982.
G. Han, S.M. Wen, H. Wang, and Q.C. Feng, Enhanced sulfidization flotation of cuprite by surface modification with hydrogen peroxide, Trans. Nonferrous Met. Soc. China, 31(2021), No. 11, p. 3564.
N.O. Lotter, D.J. Bradshaw, and A.R. Barnes, Classification of the Major Copper Sulphides into semiconductor types, and associated flotation characteristics, Miner. Eng., 96–97(2016), p. 177.
H. Wang, S. Wen, G. Han, Y. He, and Q. Feng, Adsorption behavior and mechanism of copper ions in the sulfidization flotation of malachite, Int. J. Min. Sci. Technol., 32(2022), No. 4, p. 897.
G. Han, S. Wen, H. Wang, and Q. Feng, Sulfidization regulation of cuprite by pre-oxidation using sodium hypochlorite as an oxidant, Int. J. Min. Sci. Technol., 31(2021), No. 6, p. 1117.
G. Han, S. Wen, H. Wang, and Q. Feng, Identification of copper-sulfide species on the cuprite surface and its role in sulfidization flotation, Colloid Surf. A., 624(2021), art. No. 126854.
Q.C. Feng, W.H. Yang, S.M. Wen, H. Wang, W.J. Zhao, and G. Han, Flotation of copper oxide minerals: A review, Int. J. Min. Sci. Technol., 32(2022), No. 6, p. 1351.
C. Liu, G.L. Zhu, S.X. Song, and H.Q. Li, Interaction of gangue minerals with malachite and implications for the sulfidization flotation of malachite, Colloids Surf. A, 555(2018), p. 679.
Y.F. Fu, Y. Hou, R. Wang, et al., Detailed insights into improved chlorite removal during hematite reverse flotation by sodium alginate, Miner. Eng., 173(2021), art. No. 107191.
X.R. Zhang, L. Liang, Y.H. Li, Y.G. Zhu, L. Han, and C.B. Li, Flotation separation performance of malachite from calcite with new chelating collector and its adsorption mechanism, Sep. Purif. Technol., 255(2021), art. No. 117732.
R. Liu, D. Liu, J. Li, et al., Sulfidization mechanism in malachite flotation: A heterogeneous solid-liquid reaction that yields CuxSy phases grown on malachite, Miner. Eng., 154(2020), art. No. 106420.
Z.L. Li, F. Rao, B. Guo, W.R. Zuo, S.X. Song, and A. López-Valdivieso, Effects of calcium ions on malachite flotation with octyl hydroxamate, Miner. Eng., 141(2019), art. No. 105854.
K.P. Ananthapadmanabhan and P. Somasundaran, Surface precipitation of inorganics and surfactants and its role in adsorption and flotation, Colloids Surf., 13(1985), p. 151.
A.S. Freitas, E. Matiolo, and R.T. Rodrigues, Flotation of calcite from apatite of a uranium-carbonate phosphate ore using carbon dioxide, Miner. Eng., 173(2021), art. No. 107240.
J. Liu, D.C. Kong, R.Q. Xie, Y.J. Li, Y.M. Zhu, and C. Liu, Flotation behavior and mechanism of hydroxycitric acid as a depressant on the flotation separation of cassiterite from calcite, Miner. Eng., 170(2021), art. No. 107046.
J. Ralston, D. Fornasiero, and S. Grano, Pulp and solution chemistry, [in] Froth Flotation: A Century of Innovation, Society for Mining, Metallurgy, and Exploration, Inc., Littleton, 2007, p. 227.
X. Wang, W.H. Jia, C.R. Yang, et al., Innovative application of sodium tripolyphosphate for the flotation separation of scheelite from calcite, Miner. Eng., 170(2021), art. No. 106981.
C. Liu, S. Song, H. Li, and G. Ai, Sulfidization flotation performance of malachite in the presence of calcite, Miner. Eng., 132(2019), p. 293.
Q. Zhang, Y.J. Wang, Q.C. Feng, et al., Identification of sulfidization products formed on azurite surfaces and its correlations with xanthate adsorption and flotation, Appl. Surf. Sci., 511(2020), art. No. 145594.
Y.F. Fu, W.Z. Yin, X.S. Dong, et al., New insights into the flotation responses of brucite and serpentine for different conditioning times: Surface dissolution behavior, Int. J. Miner. Metall. Mater., 28(2021), No. 12, p. 1898.
Q. Zhang, S. Wen, W. Nie, and Q. Feng, Effect of dissolved species of cerussite on quartz flotation in sulfidization xanthate system, J. Mol. Liq., 356(2022), art. No. 119055.
Q. Zhang, S. Wen, S. Zhang, and Q. Feng, Surface chemistry of dissolved species of cerussite and calcite and its effect on flotation performance, Colloids Surf. A, 646(2022), art. No. 128945.
B. Yang, Y.F. Fu, W.Z. Yin, Q.Y. Sheng, Z.L. Zhu, and X.M. Yin, Selective collection performance of an efficient quartz collector and its response to flotation separation of malachite from quartz, Miner. Eng., 172(2021), art. No. 107174.
F.X. Li, H. Zhong, H.F. Xu, H. Jia, and G.Y. Liu, Flotation behavior and adsorption mechanism of α-hydroxyoctyl phosphinic acid to malachite, Miner. Eng., 71(2015), p. 188.
Y.G. Chen, B. Feng, H.S. Yan, et al., Adsorption and depression mechanism of an eco-friendly depressant dextrin onto fluorite and calcite for the efficiency flotation separation, Colloids Surf. A, 635(2022), art. No. 127987.
H. Sun, F. Nie, and J. Zhang, Investigation on the flotation separation of smithsonite from calcite using calcium lignosulphonate as depressant, Colloids Surf. A, 630(2021), art. No. 127571.
L. Wang, W. Liu, F. Liu, J. Liu, and H. Zhang, Discrepant adsorption behavior of sodium alginate onto apatite and calcite surfaces: Implications for their selective flotation separation, Miner. Eng., 181(2022), art. No. 107553.
R.P. Liao, S.M. Wen, Q.C. Feng, J.S. Deng, and H. Lai, Activation mechanism of ammonium oxalate with pyrite in the lime system and its response to flotation separation of pyrite from arsenopyrite, Int. J. Miner. Metall. Mater., 30(2023), No. 2, p. 271.
G. Liu, Y. Huang, X. Qu, J. Xiao, X. Yang, and Z. Xu, Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1,2,4-triazole-5-thione to malachite by ToF-SIMS, XPS, FTIR, contact angle, zeta potential and micro-flotation, Colloids Surf. A, 503(2016), p. 34.
R. Liao, S. Wen, J. Liu, and Q. Feng, Flotation separation of fine smithsonite from calcite using sodium hexametaphosphate as the depressant in the Na2S-Pb(II)-KIAX system, Sep. Purif. Technol., 295(2022), art. No. 121245.
Q. Feng, M. Wang, G. Zhang, W. Zhao, and G. Han, Enhanced adsorption of sulfide and xanthate on smithsonite surfaces by lead activation and implications for flotation intensification, Sep. Purif. Technol., 307(2023), art. No. 122772.
B. Yang, W. Yin, J. Yao, Q. Sheng, and Z. Zhu, Role of decaethoxylated stearylamine in the selective flotation of hornblende and siderite: An experimental and molecular dynamics simulation study, Appl. Surf. Sci., 571(2022), art. No. 151177.
W. Zhao, M. Wang, B. Yang, Q. Feng, and D.W. Liu, Enhanced sulfidization flotation mechanism of smithsonite in the synergistic activation system of copper-ammonium species, Miner. Eng., 187(2022), art. No. 107796.
Y.L. Wang, G.C. He, D. Abudukade, et al., Selective inhibition of sodium tripolyphosphate on calcite in the process of magnesite flotation, J. Mol. Liq., 345(2022), art. No. 117412.
Acknowledgements
This work was financially supported by Yunnan Fundamental Research Projects (No. 202101BE070001-009) and National Natural Science Foundation of China (No. 51464029).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors declare no potential conflict of interest.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Shen, Z., Wen, S., Wang, H. et al. Effect of dissolved components of malachite and calcite on surface properties and flotation behavior. Int J Miner Metall Mater 30, 1297–1309 (2023). https://doi.org/10.1007/s12613-023-2606-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-023-2606-9