Abstract
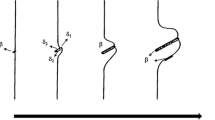
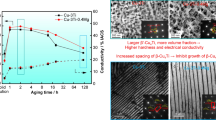
The morphology and growth kinetics of discontinuous precipitation (DP) in a Cu–20Ni–20Mn alloy were investigated in the temperature range of 523–673 K by optical microscopy, scanning electron microscopy, and transmission electron microscopy. A lamellar mixed structure consisting of alternating lamellae of a matrix and NiMn phase was observed in DP colonies. The volume fraction of regions formed by a DP reaction was determined by quantitative metallographic measurements. The kinetics of DP was evaluated on the basis of the Johnson–Mehl–Avrami–Kolmogorov equation, which resulted in a time exponent of approximately 1.5. We confirmed that the nucleation of the discontinuous precipitate was confined to grain edges or boundaries at an early stage of the reaction. The activation energy of DP process was determined to be approximately (72.7 ± 7.2) kJ/mol based on the Arrhenius equation; this result suggests that DP is controlled by grain boundary diffusion. The hardness values exhibited good correlation with the volume fraction of DP; this correlation was attributed to the presence of the ordered NiMn phase.
Similar content being viewed by others
References
A. Boegel, K. Ohla, and H.R. Mueller, Copper-Nickel-Manganese Alloy, Products Made Therefrom and Method of Manufacture of Products Therefrom, US Patent, US6811623 B2, 2004.
W.H. Sun, H.H. Xu, Y. Du, S.H. Liu, H.L. Chen, L.J. Zhang, and B.Y. Huang, Experimental investigation and thermodynamic modeling of the Cu-Mn-Ni system, Calphad, 33(2009), No. 4, p. 642.
J. Miettinen, Thermodynamic description of the Cu-Mn-Ni system at the Cu–Ni side, Calphad, 27(2003), No. 2, p. 147.
Q.H. Pan, A highly elastic Cu-20Ni-20Mn alloy, Chin. J. Nonferrous Met., 6(1996), No. 4, p. 91.
Z. Boumerzoug, L. Boudhib, and A. Chala, Influence of plastic deformation on occurrence of discontinuous precipitation, J. Mater. Sci., 40(2005), No. 12, p. 3199.
I. Manna, S.K. Pabi, and W. Gust, Discontinuous reactions in solids, Int. Mater. Rev., 46(2001), No. 2, p. 53.
R. Monzen, C. Watanabe, D. Mino, and S. Saida, Initiation and growth of the discontinuous precipitation reaction at [011] symmetric tilt boundaries in Cu-Be alloy bicrystals, Acta Mater., 53(2005), No. 4, p. 1253.
J.D. Robson, Modeling competitive continuous and discontinuous precipitation, Acta Mater., 61(2013), No. 20, p. 7781.
S. Shapiro, D.E. Tyler, and R. Lanam, Phenomenology of precipitation in Copper-20 pct Nickel-20 pct Manganese, Metall. Trans., 11(1974), No. 5, p. 2457.
D. Bradai, M. Kadi-hanifi, P. Zieba, W.M. Kuschke, and W. Gust, The kinetics of the discontinuous precipitation and dissolution in Mg-rich Al alloys, J. Mater. Sci., 34(1999), No. 21, p. 5331.
H.P. Ng, C.J. Bettles, and B.C. Muddle, Some observations on deformation-related discontinuous precipitation in an Al-14.6at.%Zn alloy, J. Alloys Compd., 509(2011), No. 5, p. 1582.
W.B. Xie, Q.S. Wang, X.J. Mi, G.L. Xie, D.M. Liu, X.C. Gao, and Y. Li, Microstructure evolution and properties of Cu-20Ni-20Mn alloy during aging process, Trans. Nonferrous Met. Soc. China, 25(2015), No. 10, p. 3247.
M.I. Barrena, J.M. Gómez de Salazar, L. Pascual, and A. Soria, Determination of the kinetic parameters in magnesium alloy using TEM and DSC techniques, J. Therm. Anal. Calorim., 113(2013), No. 2, p. 713.
J.W. Cahn, The kinetics of grain boundary nucleated reactions, Acta Metall., 4(1956), No. 5, p. 449.
H. Tsubakino and R. Nozato, Discontinuous precipitation in Cu?Mg alloys, J. Mater. Sci., 19(1984), No. 9, p. 3013.
E. Contreras-piedras, R. Esquivel-gonzalez, V.M. López-hirata, M.L. Saucedo-Muñoz, A.M. Paniagua-mercado, and H.J. Dorantes-rosales, Growth kinetics of cellular precipitation in a Mg-8.5Al-0.5Zn-0.2Mn (wt.%) alloy, Mater. Sci. Eng. A, 527(2010), No. 29-30, p. 7775.
M.B. Berkenpas, J.A. Barnard, R.V. Ramanujan, and H.I. Aaronson, A critique of activation energies for nucleation growth and overall transformation kinetics, Scripta Metall., 20(1986), No. 3, p. 323.
W.F. Gale and T.C. Totemeir, Smithells Metals Reference Book, 8th Ed., Butterworth-Heinemann, Oxford, 2004, p. 13.
I. Manna, J.N. Jha, and S.K. Pabi, Kinetics of discontinuous precipitation in a Zn-2.5at% Cu alloy, J. Mater. Sci., 30(1995), No. 6, p. 1449.
A.R. Eivani and A.K. Taheri, Modeling age hardening kinetics of an Al-Mg-Si-Cu aluminum alloy, J. Mater. Process. Technol., 205(2008), No. 1-3, p. 388.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, Wb., Wang, Qs., Xie, Gl. et al. Kinetics of discontinuous precipitation in Cu–20Ni–20Mn alloy. Int J Miner Metall Mater 23, 323–329 (2016). https://doi.org/10.1007/s12613-016-1241-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-016-1241-0