Abstract
In the face of rising antibiotic resistance and the need for novel therapeutic approaches against cancer, the present study delves into the various facets of biosynthesized silver nanoparticles (AgNPs) derived from the probiotic strain Lactobacillus casei (AgNPs-LC), assessing their efficacy in combating bacterial infections, disrupting biofilm formation, interfering with quorum sensing mechanisms, and exhibiting anti-cancer properties. The results showed that the AgNPs-LC had a spherical shape with an average size of 15 nm. The biosynthesized AgNPs-LC showed a symmetrical absorption spectrum with a peak at 458 nm with a diameter of 5–20 nm. AgNPs-LC exhibited significant antibacterial activity against Gram-positive and Gram-negative bacteria and inhibited the biofilm formation (> 50% at sub-MIC) and quorum sensing-mediated virulence factors, such as the production of violacein in C. violaceum (> 80% at sub-MIC), pyocyanin in P. aeruginosa (> 70% at sub-MIC), and prodigiosin in S. marcescens (> 80% at sub-MIC). The exopolysaccharides (EPS) were also found to reduce in the presence of AgNPs-LC. Furthermore, the AgNPs-LC showed anti-cancer and anti-metastasis activity via inhibiting cell migration and invasion of human lung cancer (A-549) cells. Overall, the present study brings out the multifaceted therapeutic capabilities of AgNPs-LC which offer exciting prospects for the development of innovative biomedical and pharmaceutical interventions, making AgNPs-LC a versatile and promising candidate for a wide range of applications in healthcare and medicine. However, further research is essential to fully harness their therapeutic potential.
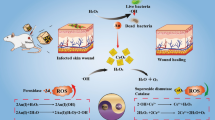
Graphical Abstract










Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adnan M, Alshammari E, Patel M, Ashraf SA, Khan S, Hadi S (2018) Significance and potential of marine microbial natural bioactive compounds against biofilms/biofouling: necessity for green chemistry. PeerJ 6:e5049
Adnan M, Patel M, Deshpande S, Alreshidi M, Siddiqui AJ, Reddy MN et al (2020) Effect of Adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: an in vitro-in silico approach. Front Microbiol 11:823
Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA et al (2018) Bacterial biofilm and associated infections. J Chin Med Assoc 81(1):7–11
Rather MA, Gupta K, Mandal M (2021) Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz J Microbiol 1–18
Abusrewil S, Alshanta OA, Albashaireh K, Alqahtani S, Nile CJ, Scott JA et al (2020) Detection, treatment and prevention of endodontic biofilm infections: what’s new in 2020? Crit Rev Microbiol 46(2):194–212
Chen L, Wen YM (2011) The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci 3(2):66–73
Evelhoch SR (2020) Biofilm and chronic nonhealing wound infections. Surg Clin 100(4):727–732
Caldara M, Belgiovine C, Secchi E, Rusconi R (2022) Environmental, microbiological, and immunological features of bacterial biofilms associated with implanted medical devices. Clin Microbiol Rev 35(2):e00221-e320
Rimondini L, Cochis A, Varoni E, Azzimonti B, Carrassi A (2014) Biofilm formation on implants and prosthetic dental materials. Handbook of bioceramics and biocomposites. Elsevier; p 1–37
Adnan M, Siddiqui AJ, Hamadou WS, Snoussi M, Badraoui R, Ashraf SA et al (2021) Deciphering the molecular mechanism responsible for efficiently inhibiting metastasis of human non-small cell lung and colorectal cancer cells targeting the matrix metalloproteinases by Selaginella repanda. Plants 10(5):979
Ganesh K, Massague J (2021) Targeting metastatic cancer. Nat Med 27(1):34–44
Jamal M, Keywan M (2021) Steps in metastasis: an updated review. Med Oncol 38(1)
Baskar R, Lee KA, Yeo R, Yeoh K-W (2012) Cancer and radiation therapy: current advances and future directions. Int J Med Sci 9(3):193
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang Z et al (2015) The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Trends 9(1):16–34
Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC et al (2021) New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med 9:20503121211034370
Adnan M, Patel M, Reddy MN, Alshammari E (2018) Formulation, evaluation and bioactive potential of Xylaria primorskensis terpenoid nanoparticles from its major compound xylaranic acid. Sci Rep 8(1):1740
Ashraf SA, Siddiqui AJ, Abd Elmoneim OE, Khan MI, Patel M, Alreshidi M et al (2021) Innovations in nanoscience for the sustainable development of food and agriculture with implications on health and environment. Sci Total Environ 768:144990
Awadelkareem AM, Siddiqui AJ, Noumi E, Ashraf SA, Hadi S, Snoussi M et al (2023) Biosynthesized silver nanoparticles derived from probiotic Lactobacillus rhamnosus (AgNPs-LR) targeting biofilm formation and quorum sensing-mediated virulence factors. Antibiotics 12(6):986
Dar TB, Bhat AR, Biteghe FAN, Bhat AR, Malindi Z (2022) Nanotechnology and nanomedicine. Fundamentals and Advances in Medical Biotechnology. Springer; p 325–61
Tarafdar J, Sharma S, Raliya R (2013) Nanotechnology: interdisciplinary science of applications. Afr J Biotechnol 12(3)
Maksimović M (2017) The roles of nanotechnology and internet of nano things in healthcare transformation. TecnoLógicas 20(40):139–153
Sagadevan S, Periasamy M (2014) Recent trends in nanobiosensors and their applications-a review. Rev Adv Mater Sci 2014(36):62–69
Sironmani A, Daniel K (2011) Silver nanoparticles–universal multifunctional nanoparticles for bio sensing, imaging for diagnostics and targeted drug delivery for therapeutic applications. Drug discovery and development–present and future. 463–84
Ali Z, Ullah R, Tuzen M, Ullah S, Rahim A, Saleh TA (2022) Colorimetric sensing of heavy metals on metal doped metal oxide nanocomposites: a review. Trends Environ Anal Chem e00187
Kumar H, Venkatesh N, Bhowmik H, Kuila A (2018) Metallic nanoparticle: a review. Biomed J Sci Tech Res 4(2):3765–3775
Mayegowda SB, Roy A, Manjula N, Pandit S, Alghamdi S, Almehmadi M et al (2023) Eco-friendly synthesized nanoparticles as antimicrobial agents: an updated review
Nikezić AVV, Bondžić AM, Vasić VM (2020) Drug delivery systems based on nanoparticles and related nanostructures. Eur J Pharm Sci 151:105412
Harish V, Tewari D, Gaur M, Yadav AB, Swaroop S, Bechelany M et al (2022) Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 12(3):457
Hasan S (2015) A review on nanoparticles: their synthesis and types. Res J Recent Sci 2277:2502
Remya V, Abitha V, Rajput PS, Rane AV, Dutta A (2017) Silver nanoparticles green synthesis: a mini review. Chem Int 3(2):165–171
Mustapha T, Misni N, Ithnin NR, Daskum AM, Unyah NZ (2022) A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int J Environ Res Public Health 19(2):674
Akhtar N, Pathak K (2017) Probiotics as a tool to biosynthesize metallic nanoparticles: research reports and patents survey. Recent Pat Drug Delivery Formulation 11(1):5–18
Adnan M, Patel M, Hadi S (2017) Functional and health promoting inherent attributes of Enterococcus hirae F2 as a novel probiotic isolated from the digestive tract of the freshwater fish Catla catla. PeerJ 5:e3085
Alshammari E, Patel M, Sachidanandan M, Kumar P, Adnan M (2019) Potential evaluation and health fostering intrinsic traits of novel probiotic strain Enterococcus durans F3 isolated from the gut of fresh water fish Catla catla. Food Sci Anim Resour 39(5):844
Hill D, Sugrue I, Tobin C, Hill C, Stanton C, Ross RP (2018) The Lactobacillus casei group: history and health related applications. Front Microbiol 2107
Mishra V, Prasad D (2005) Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int J Food Microbiol 103(1):109–115
Syame SM, Mansour AS, Khalaf DD, Ibrahim E, Gaber E (2020) Green synthesis of silver nanoparticles using lactic acid bacteria: assessment of antimicrobial activity. World’s Vet J 10:625–633
Adebayo-Tayo BC, Popoola AO (2017) Biogenic synthesis and antimicrobial activity of silver nanoparticle using exopolysaccharides from lactic acid bacteria. Int J Nano Dimens 8(1):61
Sintubin L, De Windt W, Dick J, Mast J, Van Der Ha D, Verstraete W et al (2009) Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol 84:741–749
Bazaid AS, Alsolami A, Patel M, Khateb AM, Aldarhami A, Snoussi M et al (2023) Antibiofilm, antimicrobial, anti-quorum sensing, and antioxidant activities of Saudi Sidr Honey: in vitro and molecular docking studies. Pharmaceutics 15(9):2177
Biedenbach D, Lob S, Badal R, Sahm D (2015) Variability of Susceptibility and multidrug resistance among K. pneumoniae from IAI in Asia/Pacific countries–SMART 2012–2013. Int J Antimicrob Agents PO BOX 211:1000
Matz C, Deines P, Boenigk J, Arndt H, Eberl L, Kjelleberg S et al (2004) Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl Environ Microbiol 70(3):1593–1599
Ugurlu A, Yagci AK, Ulusoy S, Aksu B, Bosgelmez-Tinaz G (2016) Phenolic compounds affect production of pyocyanin, swarming motility and biofilm formation of Pseudomonas aeruginosa. Asian Pac J Trop Biomed 6(8):698–701
Slater H, Crow M, Everson L, Salmond GP (2003) Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and-independent pathways. Mol Microbiol 47(2):303–320
Ghaima KK, Rasheed SF, Ahmed EF (2013) Antibiofilm, antibacterial and antioxidant activities of water extract of Calendula officinalis flowers. Int J Biol Pharm Res 4(7):465–470
Musthafa KS, Ravi AV, Annapoorani A, Packiavathy ISV, Pandian SK (2010) Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-acyl-homoserine lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy 56(4):333–339
Viszwapriya D, Prithika U, Deebika S, Balamurugan K, Pandian SK (2016) In vitro and in vivo antibiofilm potential of 2, 4-Di-tert-butylphenol from seaweed surface associated bacterium Bacillus subtilis against group A streptococcus. Microbiol Res 191:19–31
Lagunin A, Stepanchikova A, Filimonov D, Poroikov V (2000) PASS: prediction of activity spectra for biologically active substances. Bioinformatics 16(8):747–748
Al-Rajhi AM, Qanash H, Bazaid AS, Binsaleh NK, Abdelghany TM (2023) Pharmacological evaluation of Acacia nilotica flower extract against Helicobacter pylori and human hepatocellular carcinoma in vitro and in silico. J Funct Biomater 14(4):237
Elasbali AM, Al-Soud WA, Al-Oanzi ZH, Qanash H, Alharbi B, Binsaleh NK et al (2022) Cytotoxic activity, cell cycle inhibition, and apoptosis-inducing potential of Athyrium hohenackerianum (Lady Fern) with its phytochemical profiling. Evid Based Complement Alternat Med 2022
Wu J-G, Ma L, Lin S-H, Wu Y-B, Yi J, Yang B-J et al (2017) Anticancer and anti-angiogenic activities of extract from Actinidia eriantha Benth root. J Ethnopharmacol 203:1–10
Korbekandi H, Iravani S, Abbasi S (2012) Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. casei. J Chem Technol Biotechnol 87(7):932–937
Bairán G, Rebollar-Pérez G, Chávez-Bravo E, Torres E (2020) Treatment processes for microbial resistance mitigation: the technological contribution to tackle the problem of antibiotic resistance. Int J Environ Res Public Health 17(23):8866
Malaekeh-Nikouei B, Bazzaz BSF, Mirhadi E, Tajani AS, Khameneh B (2020) The role of nanotechnology in combating biofilm-based antibiotic resistance. J Drug Delivery Sci Technol 60:101880
Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N et al (2020) The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials 10(8):1566
Tang S, Zheng J (2018) Antibacterial activity of silver nanoparticles: structural effects. Adv Healthcare Mater 7(13):1701503
Tamboli DP, Lee DS (2013) Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram positive and gram negative bacteria. J Hazard Mater 260:878–884
Torabian P, Ghandehari F, Fatemi M (2018) Biosynthesis of iron oxide nanoparticles by cytoplasmic extracts of bacteria lactobacillus casei. Asian J Green Chem 2(3):171–280
Rajesh S, Dharanishanthi V, Kanna AV (2015) Antibacterial mechanism of biogenic silver nanoparticles of Lactobacillus acidophilus. J Exp Nanosci 10(15):1143–1152
Nithya R, Ragunathan R (2012) Synthesis of silver nanoparticles using a probiotic microbe and its antibacterial effect against multidrug resistant bacteria. Afr J Biotech 11(49):11013–11021
Xu C, Guo Y, Qiao L, Ma L, Cheng Y, Roman A (2018) Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by enterotoxigenic Escherichia coli K88. Front Microbiol 9:1129
Markus J, Mathiyalagan R, Kim Y-J, Abbai R, Singh P, Ahn S et al (2016) Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb Technol 95:85–93
Kumar KK, Mahalakshmi S, Harikrishna N, Reddy G (2016) Production, characterization and antimicrobial activity of silver nanoparticles produced by Lactobacillus amylophilus GV6. Eur J Pharm Med Res 3(7):236242
Naseer Q, Xue X, Wang X, Dang S, Din S, Jamil J (2021) Synthesis of silver nanoparticles using Lactobacillus bulgaricus and assessment of their antibacterial potential. Braz J Biol 82
Sharma S, Sharma N, Kaushal N (2022) Comparative account of biogenic synthesis of silver nanoparticles using probiotics and their antimicrobial activity against challenging pathogens. BioNanoScience 12(3):833–840
Adnan M, Siddiqui AJ, Noumi E, Ashraf SA, Awadelkareem AM, Hadi S et al (2023) Biosurfactant derived from probiotic Lactobacillus acidophilus exhibits broad-spectrum antibiofilm activity and inhibits the quorum sensing-regulated virulence. Biomol Biomed
Abdel-Fattah WI, Ali GW (2018) On the anti-cancer activities of silver nanoparticles. J Appl Biotechnol Bioeng 5(1):43–46
Moghadam BY, Hou W-C, Corredor C, Westerhoff P, Posner JD (2012) Role of nanoparticle surface functionality in the disruption of model cell membranes. Langmuir 28(47):16318–16326
Yin H, Casey PS, McCall MJ, Fenech M (2010) Effects of surface chemistry on cytotoxicity, genotoxicity, and the generation of reactive oxygen species induced by ZnO nanoparticles. Langmuir 26(19):15399–15408
Mao B-H, Tsai J-C, Chen C-W, Yan S-J, Wang Y-J (2016) Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology 10(8):1021–1040
Krishnan N, Velramar B, Ramatchandirin B, Abraham GC, Duraisamy N, Pandiyan R et al (2018) Effect of biogenic silver nanocubes on matrix metalloproteinases 2 and 9 expressions in hyperglycemic skin injury and its impact in early wound healing in streptozotocin-induced diabetic mice. Mater Sci Eng C 91:146–152
Mei ML, Li Q, Chu C, Yiu CK, Lo EC (2012) The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent Mater 28(8):903–908
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292
Kavaz D, Umar H, Shehu S (2018) Synthesis, characterization, antimicrobial and antimetastatic activity of silver nanoparticles synthesized from Ficus ingens leaf. Artif Cells Nanomed Biotechnol 46(sup3):S1193–S1203
Mordmuang A, Shankar S, Chethanond U, Voravuthikunchai SP (2015) Effects of Rhodomyrtus tomentosa leaf extract on staphylococcal adhesion and invasion in bovine udder epidermal tissue model. Nutrients 7(10):8503–8517
Barar J (2015) Bioimpacts of nanoparticle size: why it matters? BioImpacts 5(3):113
Wang W, Gaus K, Tilley RD, Gooding JJ (2019) The impact of nanoparticle shape on cellular internalisation and transport: what do the different analysis methods tell us? Mater Horiz 6(8):1538–1547
Ayala V, Herrera AP, Latorre-Esteves M, Torres-Lugo M, Rinaldi C (2013) Effect of surface charge on the colloidal stability and in vitro uptake of carboxymethyl dextran-coated iron oxide nanoparticles. J Nanopart Res 15(8):1874
Guerrini L, Alvarez-Puebla RA, Pazos-Perez N (2018) Surface modifications of nanoparticles for stability in biological fluids. Materials 11(7):1154
Javed R, Sajjad A, Naz S, Sajjad H, Ao Q (2022) Significance of capping agents of colloidal nanoparticles from the perspective of drug and gene delivery, bioimaging, and biosensing: an insight. Int J Mol Sci 23(18):10521
Barabadi H, Jounaki K, Pishgahzadeh E, Morad H, Sadeghian-Abadi S, Vahidi H et al (2022) Antiviral potential of green-synthesized silver nanoparticles. Handbook of Microbial Nanotechnology. Elsevier; p 285–310
Talank N, Morad H, Barabadi H, Mojab F, Amidi S, Kobarfard F et al (2022) Bioengineering of green-synthesized silver nanoparticles: in vitro physicochemical, antibacterial, biofilm inhibitory, anticoagulant, and antioxidant performance. Talanta 243:123374
Barabadi H, Mobaraki K, Ashouri F, Noqani H, Jounaki K, Mostafavi E (2023) Nanobiotechnological approaches in antinociceptive therapy: animal-based evidence for analgesic nanotherapeutics of bioengineered silver and gold nanomaterials. Adv Colloid Interface Sci 102917
Mostafavi E, Zarepour A, Barabadi H, Zarrabi A, Truong LB, Medina-Cruz D (2022) Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: beginning a new era in cancer theragnostic. Biotechnol Rep 34:e00714
Acknowledgements
I would like to thank and appreciate all the support and technical assistance provided by the Scientific Research Deanship at the University of Ha’il, Saudi Arabia, through project number RG-23049.
Funding
This research has been funded by the Scientific Research Deanship at the University of Ha’il, Saudi Arabia, through project number RG-23049.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.J.S., M.P., and M.A.; methodology: M.M.A., W.A, S.J., M.K., M.J.A., and A.K.; validation, R.B., M.J.A, A.A., W.A., M.K., and S.J; formal analysis: M.A., R.B., M.M.A., A.K., M.P., M.K. and W.A.; investigation, M.M.A., A.K., R.B., W.A., A.A., and S.J.; data curation, M.M.A., M.J.A., A.K., M.P., A.A., W.A., M.K., and R.B.; writing—original draft preparation, A.J.S. M.P., and M.A.; writing—review and editing, A.J.S., M.P., M.A., and S.J.; visualization, A.A., A.B. M.A., and R.B.; supervision, A.J.S.; project administration, A.J.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddiqui, A.J., Patel, M., Jahan, S. et al. Silver Nanoparticles Derived from Probiotic Lactobacillus casei—a Novel Approach for Combating Bacterial Infections and Cancer. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10201-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10201-3