Abstract
Advanced cathode materials have been considered as the key to significantly improve the energy density of lithium-ion batteries (LIBs). High-Ni layer-structured cathodes, especially with Ni atomic content above 0.9 (LiNixM1−xO2, x ≥ 0.9), exhibit high capacity to be commercially available in electric vehicles (EVs). However, the intrinsic structure instability of high-Ni materials and the negative impacts severely restrict their further application. In addition, Co has various effective efforts to stabilize the layered structure. Nevertheless, due to the high cost of Co, it is required to be replaced. Therefore, modification methods on increasing the stability of high-Ni cathode with the reduction of Co content have been widely investigated. In this review, we summarized various effective research progresses and several potential modification strategies of Co-free/Co-poor layered cathodes with Ni content over 0.9. The challenges and development opportunities of high-Ni, Co-free/Co-poor cathodes are further overviewed to meet the future commercial energy demands.
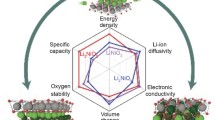
Graphical abstract

摘要
先进的正极材料被认为是显著提高锂离子电池能量密度的关键。高镍层状正极材料(特别是镍含量在0.9以上)因其高容量而具有较高的商用能力。然而,高镍层状正极材料自身的结构不稳定性及其负面影响严重限制了其进一步应用。Co元素可以用来稳定层状结构,由于钴的成本太高,因此,通过降低Co含量来提高高镍正极材料稳定性的改性方法得到了广泛的研究。本文综述了无钴/贫钴层状正极材料(镍含量大于0.9的)的研究进展和几种可能的改性策略,进一步概述了高镍、无钴/贫钴正极的挑战和发展机遇,以满足未来的商业能源需求。

Reproduced with permission from Ref. [30]. Copyright 2003, Elsevier. Reproduced with permission from Ref. [29]. Copyright 2013, American Chemical Society. Reproduced with permission from Ref. [15]. Copyright 2019, Wiley–VCH. Reproduced with permission from Ref. [31]. Copyright 2020, Wiley–VCH. Reproduced with permission from Ref. [14]. Copyright 2021, Wiley–VCH. Reproduced with permission from Ref. [28]. Copyright 2021, Wiley–VCH

Reproduced with permission from Ref. [40]. Copyright 2020, Elsevier. b Structural inhomogeneity at high C-rates existing in single-crystal cathodes. Reproduced with permission from Ref. [42]. Copyright 2021, American Chemical Society. c Schematic diagram of a Mo-modified LiNi0.815Co0.15Al0.035O2 cathode material. Reproduced with permission from Ref. [44]. Copyright 2019, American Chemical Society

Reproduced with permission from Ref. [34]. Copyright 2019, the Electrochemical Society. b Cross-sectional SEM images of NCM90 and NM90 at 30 and 60 °C with corresponding differential capacity curves (dQ/dV) after 100 cycles. Reproduced with permission from Ref. [33]. Copyright 2019, Wiley–VCH. c Differential scanning calorimetry (DSC) pattern. Reproduced with permission from Ref. [31]. Copyright 2019, Wiley–VCH

Reproduced with permission from Ref. [14]. Copyright 2021, Wiley–VCH

Similar content being viewed by others
References
Gao L, Zhu C, Li L, Zhang C, Liu J, Yu HD, Huang W. All paper-based flexible and wearable piezoresistive pressure sensor. ACS Appl Mater Inter. 2019;11(28):25034. https://doi.org/10.1021/acsami.9b07465.
Wang L, Si W, Tong Y, Hou F, Pergolesi D, Hou J, Lippert T, Dou SX, Liang J. Graphitic carbon nitride (g-C3N4)-based nanosized heteroarrays: promising materials for photoelectrochemical water splitting. Carbon Energy. 2020;2(2):223. https://doi.org/10.1002/cey2.48.
Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem Mater. 2010;22(3):587. https://doi.org/10.1021/cm901452z.
Zhao Z, Wang C, Wang H, Shen Y, Wang Q, Li S, Liu B, Zhao N, Zhong C, Hu W. A simple way to induce anode-electrolyte interface engineering through a functional composite separator for zinc-nickel batteries. Nano Energy. 2022;97:107162. https://doi.org/10.1016/j.nanoen.2022.107162.
Deng LZ, Wu F, Gao XG, Wu WP. Development of a LiFePO4-based high power lithium secondary battery for HEVs applications. Rare Met. 2020;39(12):1457. https://doi.org/10.1007/s12598-014-0316-1.
Manthiram A. A reflection on lithium-ion battery cathode chemistry. Nat Commun. 2020;11(1):1550. https://doi.org/10.1038/s41467-020-15355-0.
Chen Y, Liu Y, Zhang J, Zhu H, Ren Y, Wang W, Zhang Q, Zhang Y, Yuan Q, Chen GX, Gallington LC, Li K, Liu X, Wu J, Liu Q, Chen Y. Constructing O2/O3 homogeneous hybrid stabilizes Li-rich layered cathodes. Energy Storage Mater. 2022. https://doi.org/10.1016/j.ensm.2022.07.016.
Jung K, Yim T. Calcium- and sulfate-functionalized artificial cathode-electrolyte interphases of Ni-rich cathode materials. Rare Met. 2021;40(10):2793. https://doi.org/10.1007/s12598-021-01710-7.
Liu WJ, Sun XZ, Zhang X, Li C, Wang K, Wen W, Ma YW. Structural evolution of mesoporous graphene/LiNi1/3Co1/3Mn1/3O2 composite cathode for Li–ion battery. Rare Met. 2021;40(3):521. https://doi.org/10.1007/s12598-020-01406-4.
Wang JH, Wang Y, Guo YZ, Liu CW, Dan LL. Electrochemical characterization ofAlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application. Rare Met. 2021;40(1):78. https://doi.org/10.1007/s12598-014-0247-x.
Li WW, Zhang XJ, Si JJ, Yang J, Sun XY. TiO2-coated LiNi0.9Co0.08Al0.02O2 cathode materials with enhanced cycle performance for Li-ion batteries. Rare Met. 2021;40(7):1719. https://doi.org/10.1007/s12598-020-01483-5.
Tsai PC, Wen B, Wolfman M, Choe MJ, Pan MS, Su L, Thornton K, Cabana J, Chiang YM. Single-particle measurements of electrochemical kinetics in NMC and NCA cathodes for Li-ion batteries. Energy Environ Sci. 2018;11(4):860. https://doi.org/10.1039/C8EE00001H.
Bianchini M, Roca-Ayats M, Hartmann P, Brezesinski T, Janek J. There and back again—the journey of LiNiO2 as a cathode active material. Angew Chem Int Ed. 2019;58(31):10434. https://doi.org/10.1002/anie.201812472.
Cui Z, Xie Q, Manthiram A. A cobalt- and manganese-free high-nickel layered oxide cathode for long-life, safer lithium-ion batteries. Adv Energy Mater. 2021;11(41):2102421. https://doi.org/10.1002/aenm.202102421.
Li J, Manthiram A. A comprehensive analysis of the interphasial and structural evolution over long-term cycling of ultrahigh-nickel cathodes in lithium-ion batteries. Adv Energy Mater. 2019;9(45):1902731. https://doi.org/10.1002/aenm.201902731.
Manthiram A. An outlook on lithium ion battery technology. ACS Cent Sci. 2017;3(10):1063. https://doi.org/10.1021/acscentsci.7b00288.
Masias A, Marcicki J, Paxton WA. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 2021;6(2):621. https://doi.org/10.1021/acsenergylett.0c02584.
Xu C, Reeves PJ, Jacquet Q, Grey CP. Phase behavior during electrochemical cycling of Ni-rich cathode materials for Li-ion batteries. Adv Energy Mater. 2021;11(7):2003404. https://doi.org/10.1002/aenm.202003404.
Wang D, Kou R, Ren Y, Sun CJ, Zhao H, Zhang MJ, Li Y, Huq A, Ko JYP, Pan F, Sun YK, Yang Y, Amine K, Bai J, Chen Z, Wang F. Synthetic control of kinetic reaction pathway and cationic ordering in high-Ni layered oxide cathodes. Adv Mater. 2017;29(39):1606715. https://doi.org/10.1002/adma.201606715.
Yin S, Deng W, Chen J, Gao X, Zou G, Hou H, Ji X. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy. 2021;83:105854. https://doi.org/10.1016/j.nanoen.2021.105854.
Kim JH, Ryu HH, Kim SJ, Yoon CS, Sun YK. Degradation mechanism of highly Ni-rich Li[NixCoyMn1-x-y]O2 cathodes with x > 0.9. ACS Appl Mater Inter. 2019;11(34):30936. https://doi.org/10.1021/acsami.9b09754.
Li M, Lu J. Cobalt in lithium-ion batteries. Science. 2020;367(6481):979. https://doi.org/10.1126/science.aba9168.
Ye Z, Qiu L, Yang W, Wu Z, Liu Y, Wang G, Song Y, Zhong B, Guo X. Nickel-rich layered cathode materials for lithium-ion batteries. Chem Eur J. 2021;27(13):4249. https://doi.org/10.1002/chem.202003987.
Duan Y, Yang L, Zhang MJ, Chen Z, Bai J, Amine K, Pan F, Wang F. Insights into Li/Ni ordering and surface reconstruction during synthesis of Ni-rich layered oxides. J Mater Chem A. 2019;7(2):513. https://doi.org/10.1039/C8TA10553G.
Zhao H, Lam WYA, Sheng L, Wang L, Bai P, Yang Y, Ren D, Xu H, He X. Cobalt-free cathode materials: families and their prospects. Adv Energy Mater. 2022;12(16):2103894. https://doi.org/10.1002/aenm.202103894.
Lun Z, Ouyang B, Kwon DH, Ha Y, Foley EE, Huang TY, Cai Z, Kim H, Balasubramanian M, Sun Y, Huang J, Tian Y, Kim H, McCloskey BD, Yang W, Clément RJ, Ji H, Ceder G. Cation-disordered rocksalt-type high-entropy cathodes for Li-ion batteries. Nat Mater. 2021;20(2):214. https://doi.org/10.1038/s41563-020-00816-0.
Liu W, Oh P, Liu X, Lee MJ, Cho W, Chae S, Kim Y, Cho J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed. 2015;54(15):4440. https://doi.org/10.1002/anie.201409262.
Park NY, Ryu HH, Park GT, Noh TC, Sun YK. Optimized Ni-rich NCMA cathode for electric vehicle batteries. Adv Energy Mater. 2021;11(9):2003767. https://doi.org/10.1002/aenm.202003767.
Wang Z, Sun W, Zhu Z, Liu T, Liu W. A novel cobalt-free, CO2-stable, and reduction-tolerant dual-phase oxygen-permeable membrane. ACS Appl Mater Inter. 2013;5(21):11038. https://doi.org/10.1021/am403272z.
Makimura Y, Ohzuku T. Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries. J Power Sources. 2003;119–121:156. https://doi.org/10.1016/S0378-7753(03)00170-8.
Li W, Lee S, Manthiram A. High-nickel NMA: a cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv Mater. 2020;32(33):e2002718. https://doi.org/10.1002/adma.202002718.
Kim UH, Ryu HH, Kim JH, Mücke R, Kaghazchi P, Yoon CS, Sun YK. Microstructure-controlled Ni-rich cathode material by microscale compositional partition for next-generation electric vehicles. Adv Energy Mater. 2019;9(15):1803902. https://doi.org/10.1002/aenm.201803902.
Aishova A, Park GT, Yoon CS, Sun YK. Cobalt-free high-capacity Ni-rich layered Li[Ni0.9Mn0.1]O2 cathode. Adv Energy Mater. 2020;10(4):1903179. https://doi.org/10.1002/aenm.201903179.
Li H, Cormier M, Zhang N, Inglis J, Li J, Dahn JR. Is cobalt needed in Ni-rich positive electrode materials for lithium ion batteries? J Electrochem Soc. 2019;166(4):A429. https://doi.org/10.1149/2.1381902jes.
Mu L, Kan WH, Kuai C, Yang Z, Li L, Sun CJ, Sainio S, Avdeev M, Nordlund D, Lin F. Structural and electrochemical impacts of Mg/Mn dual dopants on the LiNiO2 cathode in Li-metal batteries. ACS Appl Mater Inter. 2020;12(11):12874. https://doi.org/10.1021/acsami.0c00111.
Jiang M, Danilov DL, Eichel RA, Notten PHL. A review of degradation mechanisms and recent achievements for Ni-rich cathode-based Li-ion batteries. Adv Energy Mater. 2021;11(48):2103005. https://doi.org/10.1002/aenm.202103005.
Wei Z, Liang C, Jiang L, Wang L, Cheng S, Peng Q, Feng L, Zhang W, Sun J, Wang Q. In-depth study on diffusion of oxygen vacancies in Li(NixCoyMnz)O2 cathode materials under thermal induction. Energy Storage Mater. 2022;47:51. https://doi.org/10.1016/j.ensm.2022.01.054.
Wan H, Liu Z, Liu G, Yi S, Yan P, Deng H, Hu W, Gao F. Unraveling TM migration mechanisms in LiNi1/3Mn1/3Co1/3O2 by modeling and experimental studies. Nano Lett. 2021;21(16):6875. https://doi.org/10.1021/acs.nanolett.1c01985.
Guo Z, Jian Z, Zhang S, Feng Y, Kou W, Ji H, Yang G. The effect of Ni oxidation state on the crystal structure and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material for highly reversible lithium storage. J Alloy Compd. 2021;882:160642. https://doi.org/10.1016/j.jallcom.2021.160642.
Fan X, Hu G, Zhang B, Ou X, Zhang J, Zhao W, Jia H, Zou L, Li P, Yang Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy. 2020;70:104450. https://doi.org/10.1016/j.nanoen.2020.104450.
Li H, Li J, Zaker N, Zhang N, Botton GA, Dahn JR. Synthesis of single crystal LiNi0.88Co0.09Al0.03O2 with a two-step lithiation method. J Electrochem Soc. 2019;166(10):A1956. https://doi.org/10.1149/2.0681910jes.
Ryu HH, Namkoong B, Kim JH, Belharouak I, Yoon CS, Sun YK. Capacity fading mechanisms in Ni-rich single-crystal NCM cathodes. ACS Energy Lett. 2021;6(8):2726. https://doi.org/10.1021/acsenergylett.1c01089.
Bi Y, Tao J, Wu Y, Li L, Xu Y, Hu E, Wu B, Hu J, Wang C, Zhang JG, Qi Y, Xiao J. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science. 2020;370(6522):1313. https://doi.org/10.1126/science.abc3167.
Xu C, Xiang W, Wu Z, Xu Y, Li Y, Wang Y, Xiao Y, Guo X, Zhong B. Highly stabilized Ni-rich cathode material with Mo induced epitaxially grown nanostructured hybrid surface for high-performance lithium-ion batteries. ACS Appl Mater Inter. 2019;11(18):16629. https://doi.org/10.1021/acsami.9b03403.
Wu YP, Rahm E, Holze R. Effects of heteroatoms on electrochemical performance of electrode materials for lithium ion batteries. Electrochim Acta. 2002;47(21):3491. https://doi.org/10.1016/S0013-4686(02)00317-1.
Weigel T, Schipper F, Erickson EM, Susai FA, Markovsky B, Aurbach D. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 2019;4(2):508. https://doi.org/10.1021/acsenergylett.8b02302.
Liu A, Zhang N, Stark JE, Arab P, Li H, Dahn JR. Synthesis of Co-free Ni-rich single crystal positive electrode materials for lithium ion batteries: part I. Two-step lithiation method for Al- or Mg-doped LiNiO2. J Electrochem Soc. 2021;168(4):040531. https://doi.org/10.1149/1945-7111/abf7e8.
Li N, Sallis S, Papp JK, McCloskey BD, Yang W, Tong W. Correlating the phase evolution and anionic redox in Co-free Ni-rich layered oxide cathodes. Nano Energy. 2020;78:105365. https://doi.org/10.1016/j.nanoen.2020.105365.
Yoon WS, Paik Y, Yang XQ, Balasubramanian M, McBreen J, Grey CP. Investigation of the local structure of the LiNi0.5Mn0.5O2 cathode material during electrochemical cycling by X-ray absorption and NMR spectroscopy. Electrochem Solid-State Lett. 2002;5(11):A263. https://doi.org/10.1149/1.1513001.
Yang H, Wu HH, Ge M, Li L, Yuan Y, Yao Q, Chen J, Xia L, Zheng J, Chen Z, Duan J, Kisslinger K, Zeng XC, Lee WK, Zhang Q, Lu J. Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries. Adv Funct Mater. 2019;29(13):1808825. https://doi.org/10.1002/adfm.201808825.
Yang H, Li L, Liu C, Chen J, Xia L, Liu Z, Chen J, Chen Z, Duan J. Simultaneous synthesis and synergetic stabilization of Zr-doped and Li6Zr2O7-coated Ni-rich layered cathode for advanced lithium ion batteries. Electrochim Acta. 2020;364:137120. https://doi.org/10.1016/j.electacta.2020.137120.
Zhang SS. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020;24:247. https://doi.org/10.1016/j.ensm.2019.08.013.
Choi JU, Voronina N, Sun YK, Myung ST. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: yesterday, today, and tomorrow. Adv Energy Mater. 2020;10(42):2002027. https://doi.org/10.1002/aenm.202002027.
Guo YJ, Zhang CH, Xin S, Shi JL, Wang WP, Fan M, Chang YX, He WH, Wang E, Zou YG, Yang XA, Meng F, Zhang YY, Lei ZQ, Yin YX, Guo YG. Competitive doping chemistry for nickel-rich layered oxide cathode materials. Angew Chem Int Ed. 2022;61(21):e202116865. https://doi.org/10.1002/anie.202116865.
Du F, Zhou Q, Cao H, Dai H, Hu D, Sun P, Adkins J, Zheng J. Confined growth of primary grains towards stabilizing integrated structure of Ni-rich materials. J Power Sources. 2020;478:228737. https://doi.org/10.1016/j.jpowsour.2020.228737.
Meng YS, Ceder G, Grey CP, Yoon WS, Jiang M, Bréger J, Shao-Horn Y. Cation ordering in layered O3Li[NixLi1/3-2x/3Mn2/3-x/3]O2 (0 ≤ x ≤ 1/2) compounds. Chem Mater. 2005;17(9):2386. https://doi.org/10.1021/cm047779m.
Liu H, Yang R, Yang W, Bai C, Li YC, Wang G, Liu Y, Xiang W, Wu Z, Guo X. Suppressing capacity fading and voltage decay of Ni-rich cathode material by dual-ion doping for lithium-ion batteries. J Mater Sci. 2021;56(3):2347. https://doi.org/10.1007/s10853-020-05246-6.
Albrecht S, Kümpers J, Kruft M, Malcus S, Vogler C, Wahl M, Wohlfahrt-Mehrens M. Electrochemical and thermal behavior of aluminum- and magnesium-doped spherical lithium nickel cobalt mixed oxides Li1−x(Ni1−y−zCoyMz)O2 (M = Al, Mg). J Power Sources. 2003;119–121:178. https://doi.org/10.1016/S0378-7753(03)00175-7.
Dou S, Xu J, Cui X, Liu W, Zhang Z, Deng Y, Hu W, Chen Y. High-temperature shock enabled nanomanufacturing for energy-related applications. Adv Energy Mater. 2020;10(33):2001331. https://doi.org/10.1002/aenm.202001331.
Liu S, Hu Z, Wu Y, Zhang J, Zhang Y, Cui B, Liu C, Hu S, Zhao N, Han X, Cao A, Chen Y, Deng Y, Hu W. Dislocation-strained IrNi alloy nanoparticles driven by thermal shock for the hydrogen evolution reaction. Adv Mater. 2020;32(48):2006034. https://doi.org/10.1002/adma.202006034.
Liu S, Shen Y, Zhang Y, Cui B, Xi S, Zhang J, Xu L, Zhu S, Chen Y, Deng Y, Hu W. Extreme environmental thermal shock induced dislocation-rich Pt nanoparticles boosting hydrogen evolution reaction. Adv Mater. 2021;34(2):2106973. https://doi.org/10.1002/adma.202106973.
Liu Z, Duan C, Dou S, Yuan Q, Xu J, Liu WD, Chen Y. Ultrafast porous carbon activation promises high-energy density supercapacitors. Small. 2022;18(23):2200954. https://doi.org/10.1002/smll.202200954.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 22109091 and 91963113).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, ZD., Wang, CY., Zhang, JC. et al. Co-free/Co-poor high-Ni cathode for high energy, stable and low-cost lithium-ion batteries. Rare Met. 42, 2214–2225 (2023). https://doi.org/10.1007/s12598-022-02252-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02252-2