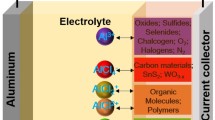
Abstract
Aluminum-ion batteries (AIBs) are recognized as one of the promising candidates for future energy storage devices due to their merits of cost-effectiveness, high voltage, and high-power operation. Many efforts have been devoted to the development of cathode materials, and the progress has been well summarized in this review paper. Moreover, in addition to materials, the intercalation mechanism also plays a key role in determining cell performance. Here, the research progress of cathode materials and corresponding ion intercalation mechanism in AIBs are summarized, including intercalation of AlCl4−, intercalation of Al3+, and coordination of AlCl2+/AlCl2+. This minireview provides comprehensive guidance on the design of cathode materials for the development of high-performance AIBs.
Graphic abstract

摘要
铝离子电池具有成本低廉, 高工作电压以及高功率密度等优势, 被认为是下一代储能器件的有力竞争者。 近些年来, 为了进一步提升铝离子电池性能, 全球各地的研究者相继研发了众多正极材料, 本文聚焦不同种类的正极材料, 详细阐述了其最新的研发进展。 此外, 不仅限于正极材料, 离子嵌入机理对电池性能也起到了至关重要的作用, 因此, 本文还根据不同种类的正极材料总结了相应的离子嵌入机理, 主要包括AlCl4-阴离子团, Al3+离子以及AlCl2+/AlCl2+离子团的嵌入脱出理论, 从而为高性能铝离子电池的正极材料设计提供前瞻性的指导。



Reproduced with permission from Ref. [10]. Copyright, 2015, Springer Nature

Reproduced with permission from Ref. [35]. Copyright 2021, Elsevier

Reproduced with permission from Ref. [40]. Copyright 2013, Springer Nature

Reproduced with permission from Ref. [48]. Copyright 2019, Springer Nature

Reproduced with permission from Ref. [52]. Copyright 2021, Springer Nature

Similar content being viewed by others
References
Durmus YE, Zhang H, Baakes F, Desmaizieres G, Hayun H, Yang L, Kolek M, Küpers V, Janek J, Mandler D. Side by side battery technologies with lithium-ion based batteries. Adv Energy Mater. 2020;10(24):2000089.
Kubota K, Dahbi M, Hosaka T, Kumakura S, Komaba S. Towards K-ion and Na-ion batteries as “Beyond Li-Ion.” Chem Rec. 2018;18(4):459.
Ponrouch A, Bitenc J, Dominko R, Lindahl N, Johansson P, Palacín MR. Multivalent rechargeable batteries. Energy Storage Mater. 2019;20:253.
Gao XL, Liu XH, Xie WL, Zhang LS, Yang SC. Multiscale observation of Li plating for lithium-ion batteries. Rare Met. 2021;40(11):3038.
Perveen T, Siddiq M, Shahzad N, Ihsan R, Ahmad A, Shahzad MI. Prospects in anode materials for sodium ion batteries—a review. Renew Sustain Energy Rev. 2020;119:109549.
Sha M, Liu L, Zhao H, Lei Y. Anode materials for potassium-ion batteries: current status and prospects. Carbon Energy. 2020;2(3):350.
Zhu L, Yang XX, Xiang YH, Kong P, Wu XW. Neurons-system-like structured SnS2/CNTs composite for high-performance sodium-ion battery anode. Rare Met. 2021;40(6):1383.
Aurbach D, Berthelot R, Ponrouch A, Salama M, Shterenberg I. Battery Systems Based on Multivalent Metals and Metal Ions. In: Monconduit L, Croguennec L, editors. Prospects for Li-Ion Batteries and Emerging Energy Electrochemical Systems. Paris: World Scientific; 2018. 237.
Bucur CB, Gregory T, Oliver AG, Muldoon J. Confession of a magnesium battery. J Phys Chem Lett. 2015;6(18):3578.
Lin MC, Gong M, Lu B, Wu Y, Wang DY, Guan M, Angell M, Chen C, Yang J, Hwang BJ, Dai H. An ultrafast rechargeable aluminium-ion battery. Nature. 2015;520(7547):324.
Jafarian M, Mahjani MG, Gobal F, Danaee I. Electrodeposition of aluminum from molten AlCl3–NaCl–KCl mixture. J Appl Electrochem. 2006;36(10):1169.
Jayaprakash N, Das SK, Archer LA. The rechargeable aluminum-ion battery. Chem Commun. 2011;47(47):12610.
Wu C, Gu S, Zhang Q, Bai Y, Li M, Yuan Y, Wang H, Liu X, Yuan Y, Zhu N, Wu F, Li H, Gu L, Lu J. Electrochemically activated spinel manganese oxide for rechargeable aqueous aluminum battery. Nat Commun. 2019;10(1):73.
Pan W, Wang Y, Zhang Y, Kwok HYH, Wu M, Zhao X, Leung DYC. A low-cost and dendrite-free rechargeable aluminium-ion battery with superior performance. J Mater Chem A. 2019;7(29):17420.
Pan W, Mao J, Wang Y, Zhao X, Leong KW, Luo S, Chen Y, Leung DYC. High-performance MnO2/Al battery with in situ electrochemically reformed AlxMnO2 nanosphere cathode. Small Methods. 2021. https://doi.org/10.1002/smtd.202100491.
Xu X, Hui KS, Hui KN, Shen J, Zhou G, Liu J, Sun Y. Engineering strategies for low-cost and high-power density aluminum-ion batteries. Chem Eng J. 2021;418(41):129385.
Wei TT, Peng P, Qi SY, Zhu YR, Yi TF. Advancement of technology towards high-performance non-aqueous aluminum-ion batteries. J Energy Chem. 2021;57:169.
Sun H, Wang W, Yu Z, Yuan Y, Wang S, Jiao S. A new aluminium-ion battery with high voltage, high safety and low cost. Chem Commun. 2015;51(59):11892.
Song Y, Jiao S, Tu J, Wang J, Liu Y, Jiao H, Mao X, Guo Z, Fray DJ. A long-life rechargeable Al ion battery based on molten salts. J Mater Chem A. 2017;5(3):1282.
Jiao H, Wang C, Tu J, Tian D, Jiao S. A rechargeable Al-ion battery: Al/molten AlCl3–urea/graphite. Chem Commun. 2017;53(15):2331.
Li C, Dong S, Tang R, Ge X, Zhang Z, Wang C, Lu Y, Yin L. Heteroatomic interface engineering in MOF-derived carbon heterostructures with built-in electric-field effects for high performance Al-ion batteries. Energy Environ Sci. 2018;11(11):3201.
Zhang C, He R, Zhang J, Hu Y, Wang Z, Jin X. Amorphous carbon-derived nanosheet-bricked porous graphite as high-performance cathode for aluminum-ion batteries. ACS Appl Mater Interfaces. 2018;10(31):26510.
Wu MS, Xu B, Chen LQ, Ouyang CY. Geometry and fast diffusion of AlCl4 cluster intercalated in graphite. Electrochim Acta. 2016;195:158.
Yu X, Wang B, Gong D, Xu Z, Lu B. Graphene nanoribbons on highly porous 3D graphene for high-capacity and ultrastable Al-ion batteries. Adv Mater. 2017;29(4):1604118.
Chen H, Chen C, Liu Y, Zhao X, Ananth N, Zheng B, Peng L, Huang T, Gao W, Gao C. High-quality graphene microflower design for high-performance Li–S and Al-ion batteries. Adv Energy Mater. 2017;7(17):1700051.
Chen H, Xu H, Wang S, Huang T, Xi J, Cai S, Guo F, Xu Z, Gao W, Gao C. Ultrafast all-climate aluminum-graphene battery with quarter-million cycle life. Sci Adv. 2017;3(12):eaao7233.
Childress AS, Parajuli P, Zhu J, Podila R, Rao AM. A Raman spectroscopic study of graphene cathodes in high-performance aluminum ion batteries. Nano Energy. 2017;39:69.
Muñoz-Torrero D, Palma J, Marcilla R, Ventosa E. Al-ion battery based on semisolid electrodes for higher specific energy and lower cost. ACS Appl Energy Mater. 2020;3(3):2285.
Jiao H, Wang J, Tu J, Lei H, Jiao S. Aluminum-ion asymmetric supercapacitor incorporating carbon nanotubes and an ionic liquid electrolyte: Al/AlCl3-[EMIm] Cl/CNTs. Energy Technol. 2016;4(9):1112.
Stadie NP, Wang S, Kravchyk KV, Kovalenko MV. Zeolite-templated carbon as an ordered microporous electrode for aluminum batteries. ACS Nano. 2017;11(2):1911.
Han M, Lv Z, Hou L, Zhou S, Cao H, Chen H, Zhou Y, Meng C, Du H, Cai M, Bian Y, Lin MC. Graphitic multi-walled carbon nanotube cathodes for rechargeable Al-ion batteries with well-defined discharge plateaus. J Power Sources. 2020;451:227769.
Jiao S, Lei H, Tu J, Zhu J, Wang J, Mao X. An industrialized prototype of the rechargeable Al/AlCl3-[EMIm] Cl/graphite battery and recycling of the graphitic cathode into graphene. Carbon. 2016;109:276.
Yang W, Lu H, Cao Y, Xu B, Deng Y, Cai W. Flexible free-standing MoS2/carbon nanofibers composite cathode for rechargeable aluminum-ion batteries. ACS Sustain Chem Eng. 2019;7(5):4861.
Hu Y, Luo B, Ye D, Zhu X, Lyu M, Wang L. An innovative freeze-dried reduced graphene oxide supported SnS2 cathode active material for aluminum-ion batteries. Adv Mater. 2017;29(48):1606132.
Zhang Y, Zhang B, Li J, Liu J, Huo X, Kang F. SnSe nano-particles as advanced positive electrode materials for rechargeable aluminum-ion batteries. Chem Eng J. 2021;403:126377.
Hudak NS. Chloroaluminate-doped conducting polymers as positive electrodes in rechargeable aluminum batteries. J Phys Chem C. 2014;118(10):5203.
Geng L, Lv G, Xing X, Guo J. Reversible electrochemical intercalation of aluminum in Mo6S8. Chem Mater. 2015;27(14):4926.
Li Z, Niu B, Liu J, Li J, Kang F. Rechargeable aluminum-ion battery based on MoS2 microsphere cathode. ACS Appl Mater Interfaces. 2018;10(11):9451.
Le D, Passerini S, Coustier F, Guo J, Soderstrom T, Owens B, Smyrl W. Intercalation of polyvalent cations into V2O5 aerogels. Chem Mater. 1998;10(3):682.
Wang W, Jiang B, Xiong W, Sun H, Lin Z, Hu L, Tu J, Hou J, Zhu H, Jiao S. A new cathode material for super-valent battery based on aluminium ion intercalation and deintercalation. Sci Rep. 2013;3:3383.
Chiku M, Takeda H, Matsumura S, Higuchi E, Inoue H. Amorphous vanadium oxide/carbon composite positive electrode for rechargeable aluminum battery. ACS Appl Mater Interfaces. 2015;7(44):24385.
Kaveevivitchai W, Huq A, Wang S, Park MJ, Manthiram A. Rechargeable aluminum-ion batteries based on an open-tunnel framework. Small. 2017;13(34):1701296.
Koketsu T, Ma J, Morgan BJ, Body M, Legein C, Dachraoui W, Giannini M, Demortière A, Salanne M, Dardoize F, Groult H, Borkiewicz OJ, Chapman KW, Strasser P, Dambournet D. Reversible magnesium and aluminium ions insertion in cation-deficient anatase TiO2. Nat Mater. 2017;16:1142.
Wang D, Cai P, Zou GQ, Hou HS, Ji XB, Tian Y, Long Z. Ultra-stable carbon-coated sodium vanadium phosphate as cathode material for sodium-ion battery. Rare Met. 2021. https://doi.org/10.1007/s12598-021-01743-y.
Jahnke T, Raafat L, Hotz D, Knöller A, Diem AM, Bill J, Burghard Z. Highly porous free-standing rGO/SnO2 Pseudocapacitive cathodes for high-rate and long-cycling Al-ion batteries. Nanomaterials. 2020;10(10):2024.
VahidMohammadi A, Hadjikhani A, Shahbazmohamadi S, Beidaghi M. Two-dimensional vanadium carbide (MXene) as a high-capacity cathode material for rechargeable aluminum batteries. ACS Nano. 2017;11(11):11135.
Zhang K, Lee TH, Bubach B, Jang HW, Ostadhassan M, Choi J-W, Shokouhimehr M. Graphite carbon-encapsulated metal nanoparticles derived from Prussian blue analogs growing on natural loofa as cathode materials for rechargeable aluminum-ion batteries. Sci Rep. 2019;9(1):13665.
Kim DJ, Yoo D-J, Otley MT, Prokofjevs A, Pezzato C, Owczarek M, Lee SJ, Choi JW, Stoddart JF. Rechargeable aluminium organic batteries. Nat Energy. 2019;4(1):51.
Bitenc J, Lindahl N, Vizintin A, Abdelhamid ME, Dominko R, Johansson P. Concept and electrochemical mechanism of an Al metal anode-organic cathode battery. Energy Storage Mater. 2020;24:379.
Zhou J, Yu X, Zhou J, Lu B. Polyimide/metal-organic framework hybrid for high performance Al-organic battery. Energy Storage Mater. 2020;31:58.
Fan X, Wang F, Ji X, Wang R, Gao T, Hou S, Chen J, Deng T, Li X, Chen L. A universal organic cathode for ultrafast lithium and multivalent metal batteries. Angew Chem. 2018;130(24):7264.
Yoo D-J, Heeney M, Glöcklhofer F, Choi JW. Tetradiketone macrocycle for divalent aluminium ion batteries. Nat Commun. 2021;12(1):2386.
Zhang X, Tang Y, Zhang F, Lee CS. A novel aluminum-graphite dual-ion battery. Adv Energy Mater. 2016;6(11):1502588.
Zhang E, Cao W, Wang B, Yu X, Wang L, Xu Z, Lu B. A novel aluminum dual-ion battery. Energy Storage Mater. 2018;11:91.
Acknowledgements
This study was financially supported by the National key R&D Program of China (No. 2018YFB0104001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pan, WD., Liu, C., Wang, MY. et al. Non-aqueous Al-ion batteries: cathode materials and corresponding underlying ion storage mechanisms. Rare Met. 41, 762–774 (2022). https://doi.org/10.1007/s12598-021-01860-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01860-8