Abstract
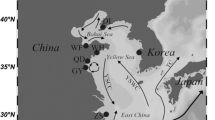
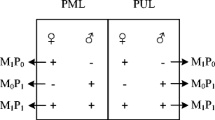
The origin and maintenance of sexual gametogenesis might provide insights into eukaryote evolution. Epigenetic variation of eukaryote well-being would provide clues that could relate ecological and evolutionary processes. To investigate the relationship between gametogenic variability and epigenetic polymorphism in the Pacific oyster Crassostrea gigas, DNA methylation differences were represented by analyzing genome-wide methylation patterns using the fluorescent-labeled methylation sensitive-amplified polymorphism (F-MSAP) technique. The C. gigas genome was widely methylated and marked variations in the pattern of methylation were shown during female and male gonad development. Statistically, the total methylation level increased from early developing stage to resorbing stage, from 28.86 to 34.53% in ovaries, while it increased from 31.20 to 35.46% in this period in testes. The unweighted pair group method with arithmetic mean and principal coordinate analysis separated the C. gigas during female and male gonad development remarkably well, indicating remarkable differences across gametogenesis between ovaries and testes. Variation was higher in testes than in ovaries by the frequency based and multivariate analyses above. Both multivariate analyses and a significantly different population epigenetic structure suggested that a striking difference might exist across gametogenesis, reflecting a turning point of the methylation mechanism during reproductive progress. This study provides some information on the role of DNA methylation in the potential mechanism during female and male gonad development.




Similar content being viewed by others
References
Ahmed M, Sparks AK (1967) A preliminary study of chromosomes of two species of oysters (Ostrea lurida and Crassostrea gigas). J Fish Res Board Can 24:2155–2159
Bonin A, Ehrich D, Manel S (2007) Statistical analysis of amplified fragment length polymorphism data: a toolbox for molecular ecologists and evolutionists. Mol Ecol 16:3737–3758
Caudy M, Vässin H, Brand M, Tuma R, Jah LY, Jan YN (1988) Daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell 55:1061–1067
Deng CM (2016) Studies on reproductive biology and physiological parameters of golden shell color strain of the Pacific oyster Crassostrea gigas. MA thesis, Ocean University of China, Qingdao
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Dheilly NM, Lelong C, Huvet A, Kellner K, Dubos MP, Riviere G, Boudry P, Favrel P (2012) Gametogenesis in the Pacific oyster Crassostrea gigas: a microarrays-based analysis identifies sex and stage specific genes. PLoS ONE 7:e36353
Díaz-Freije E, Gestal C, Castellanos-Martínez S, Morán P (2014) The role of DNA methylation on Octopus vulgaris development and their perspectives. Front Physiol 5:62
Dridi S, Romdhane MS, Elcafsi M (2007) Seasonal variation in weight and biochemical composition of the Pacific oyster, Crassostrea gigas in relation to the gametogenic cycle and environmental conditions of the Bizert lagoon, Tunisia. Aquaculture 263:238–248
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Feil R, Fraga MF (2012) Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 13:97–109
Gavery MR, Roberts SB (2014) A context dependent role for DNA methylation in bivalves. Brief Funct Genomics 13:217–222
Guo XM, Hedgecock D, Hershberger WK, Cooper K, Allen SK (1998) Genetic determinants of protandric sex in the Pacific oyster, Crassostrea gigas Thunberg. Evolution 52:394–402
Jiang Q, Li Q, Yu H, Kong LF (2016) Inheritance and variation of genomic DNA methylation in diploid and triploid Pacific oyster (Crassostrea gigas). Mar Biotechnol 18:124–132
Keyte AL, Percifield R, Liu B, Wendel JF (2006) Infraspecific DNA methylation polymorphism in cotton (Gossypium hirsutum L.). J Hered 97:444–450
Klose RJ, Bird AP (2006) Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 31:89–97
La Salle S, Mertineit C, Taketo T, Moens PB, Bestor TH, Trasler JM (2004) Windows for sex-specific methylation marked by DNA methyltransferase expression profiles in mouse germ cells. Dev Biol 268:403–415
Li Q, Liu W, Shirasu K, Chen W, Jiang S (2006) Reproductive cycle and biochemical composition of the Zhe oyster Crassostrea plicatula Gmelin in an eastern coastal bay of China. Aquaculture 261:752–759
Naimi A, Martinez AS, Specq ML, Mrac A, Diss B, Mathieu M, Sourdaine P (2009a) Identification and expression of a factor of the DM family in the oyster Crassostrea gigas. Comp Biochem Phys A 152:189–196
Naimi A, Martinez AS, Specq ML, Diss B, Mathieu M, Sourdaine P (2009b) Molecular cloning and gene expression of Cg-Foxl2 during the development and the adult gametogenetic cycle in the oyster Crassostrea gigas. Comp Biochem Phys B 154:134–142
Nicotra AB, Segal DL, Hoyle GL, Schrey AW, Verhoeven KJ, Richards CL (2015) Adaptive plasticity and epigenetic variation in response to warming in an Alpine plant. Ecol Evol 5:634–647
Olson CE, Roberts SB (2014) Genome-wide profiling of DNA methylation and gene expression in Crassostrea gigas male gametes. Front Physiol 5:224
Pérez-Figueroa A (2013) MSAP: a tool for the statistical analysis of methylation—sensitive amplified polymorphism data. Mol Ecol Resour 13:522–527
Ponger LC, Li WH (2005) Evolutionary diversification of DNA methyltransferases in eukaryotic genomes. Mol Biol Evol 22:1119–1128
Rajasethupathy P, Antonov I, Sheridan R, Frey S, Sander C, Tuschl T, Kandel ER (2012) A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell 149:693–707
Razin A, Shemer R (1995) DNA methylation in early development. Hum Mol Genet 4:1751–1755
Reik W (2007) Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447:425–432
Reyna-López GE, Simpson J, Ruiz-Herrera J (1997) Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphisms. Mol Gen Genet 253:703–710
Riviere G, Wu GC, Fellous A, Goux D, Sourdaine P, Favrel P (2013) DNA methylation is crucial for the early development in the oyster C. gigas. Mar Biotechnol 15:739–753
Roberts SB, Gavery MR (2012) Is there a relationship between DNA methylation and phenotypic plasticity in invertebrates? Front Physiol 2:116
Sanford JP, Clark HJ, Chapman VM, Rossant J (1987) Differences in DNA methylation during oogenesis and spermatogenesis and their persistence during early embryogenesis in the mouse. Gene Dev 1:1039–1046
Strömqvist M, Tooke N, Brunström B (2010) DNA methylation levels in the 5′ flanking region of the vitellogenin I gene in liver and brain of adult zebrafish (Danio rerio)—sex and tissue differences and effects of 17α-ethinylestradiol exposure. Aquat Toxicol 98:275–281
Torres-Maldonado LC, Landa PA, Moreno MN, Marmolejo VA, Meza MA, Merchant LH (2002) Expression profiles of Dax1, Dmrt1, and Sox9 during temperature sex determination in gonads of the sea turtle Lepidochelys olivacea. Gen Comp Endocrinol 129:20–26
Trautner JH, Reiser S, Blancke T, Unger K, Wysujack K (2017) Metamorphosis and transition between developmental stages in European eel (Anguilla anguilla, L.) involve epigenetic changes in DNA methylation patterns. Comp Biochem Physiol D 22:139–145
Wilhelm D, Palmer S, Koopman P (2007) Sex determination and gonadal development in mammals. Physiol Rev 87:1–28
Xu ML, Li XQ, Korban SS (2000) AFLP-Based detection of DNA methylation. Plant Mol Biol Rep 18:361–368
Yi SV, Goodisman MAD (2009) Computational approaches for understanding the evolution of DNA methylation in animals. Epigenetics 4:551–556
Zhang WL, Wang XE, Yu Q, Ming R, Jiang JM (2008) DNA methylation and heterochromatinization in the male-specific region of the primitive Y chromosome of papaya. Genome Res 18:1938–1943
Acknowledgements
This study was supported by Grants from the National Natural Science Foundation of China (31772843), Shandong Seed Project, Shandong Province (2016ZDJS06A06), major project for Tianjin seed technology (15ZXZYNC00050), and Fundamental Research Funds for the Central Universities (201762014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Li, Q., Kong, L. et al. DNA methylation frequency and epigenetic variability of the Pacific oyster Crassostrea gigas in relation to the gametogenesis. Fish Sci 84, 789–797 (2018). https://doi.org/10.1007/s12562-018-1214-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-018-1214-5