Abstract
Environmental monitoring is critical in a developing country like Egypt where there is an insufficient framework for recording and tracking outbreaks. In this study, the prevalence of human adenovirus (HAdV), rotavirus group A (RVA) was determined in urban sewage, activated sludge, drainage water, drainage sediment, Nile water, and Nile sediment, using quantitative polymerase chain reaction (qPCR) analysis. HAdV was detected in 50% of urban sewage with viral concentrations ranging from 103 to 107 genome copies/liter (GC/L), 33% of activated sludge with viral concentrations ranging from 103 to 107 GC/kilogram (GC/kg), 95% of drainage water with viral concentrations ranging from 103 to 107 GC/L, 75% of drainage sediment with viral concentrations ranging from 103 to 107 GC/L, 50% of Nile water with viral concentrations ranging from 103 to 107 GC/L, and 45% of Nile sediment with viral concentrations ranging from 103 to 107 GC/kg. RVA was detected in 50% of urban sewage with viral concentrations ranging from 103 to 107 GC/L, 75% of activated sludge with viral concentrations ranging from 103 to 107 GC/L, 58% of drainage water with viral concentrations ranging from 103 to 107 GC/L, 50% of drainage sediment with viral concentrations ranging from 103 to 107 GC/L, and 45% of Nile water with viral concentrations ranging from 103 to 107 GC/kg. In conclusion, Abu-Rawash WWTP acts as a source of HAdV and RVA, releasing them into El-Rahawy drain then to the River Nile Rosetta branch.



Similar content being viewed by others
References
Amenu, D. (2014). Wastewater treatment plants as a source of microbial pathogens in receiving watersheds. Research Journal of Chemical and Environmental Sciences, 2(6), 11–19.
Anthony, I. O., Sibanda, T., & Gusha, S. S. (2010). Inadequately treated wastewater as a source of human enteric viruses in the environment. International Journal of Environmental Research and Public Health, 7, 2620–2637.
Assis, A., Cruz, L., Ferreira, A., Bessa, M., Pinto, M., Vieira, C., et al. (2015). Relationship between viral detection and turbidity in a watershed contaminated with group A rotavirus. Environmental Science and Pollution Research, 22(9), 6886–6897.
Attoui, H., Mertens, P. P. C., Becnel, J., Belaganahalli, S., Bergoin, M., Brussaard, C. P., et al. (2012). Family reoviridae. In A. M. Q. King, M. J. Adams, E. B. Carstens, & E. J. Lefkowitz (Eds.), Virus taxonomy: Classification and nomenclature: Ninth report of the international committee on taxonomy of viruses (pp. 541–637). San Diego, California: Elsevier Academic Press.
Barcelo, D., & Kostianoy, A.G. (2011). The handbook of environmental chemistry, founded by Otto Hutzinger Editors-in-Chief: Volume 14.
Barril, P. A., Fumian, T. M., Prez, V. E., Gil, P. I., Martínez, L. C., Giordano, M. O., et al. (2015). Rotavirus seasonality in urban sewage from Argentina: Effect of meteorological variables on the viral load and the genetic diversity. Environmental Research, 138, 409–415. https://doi.org/10.1016/j.envres.2015.03.004.
Binder, A. M., Biggs, H. M., Haynes, A. K., Chommanard, C., Lu, X., Erdman, D. D., et al. (2017). Human adenovirus surveillance—United States, 2003–2016. MMWR Morbidity and Mortality Weekly Report, 66, 1039–1042.
Bofill-Mas, S., Calgua, B., Clemente-Casares, P., La Rosa, G., Iaconelli, M., Michele, M., et al. (2010). Quantification of human adenovirus in European recreational Waters. Food and Environmental Virology, 2, 101–109.
Bosch, A. (1998). Human enteric viruses in the water environment: A minireview. International Microbiology, 1(3), 191–196.
Carducci, A., Battistini, R., Rovini, E., & Verani, M. (2009). Viral removal by wastewater treatment: Monitoring of indicators and pathogens. Food and Environmental Virology, 1, 85–91.
Carducci, A., & Verani, M. (2013). Effects of bacterial, chemical, physical and meteorological variables on virus removal by a wastewater treatment plant. Food and Environmental Virology, 5, 69–76.
Chapron, C. D., Ballester, N. A., Fontaine, J. H., Frades, C. N., & Margolin, A. B. (2000). Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Applied and Environmental Microbiology, 66, 2520–2525.
Chigor, V. N., & Okoh, A. I. (2012). Quantitative RT-PCR detection of hepatitis A virus, rotaviruses and enteroviruses in the Buffalo River and source water dams in the Eastern Cape Province of South Africa. International Journal of Environmental Research and Public Health, 9(11), 4017–4032.
Da Silva, M., Victoria, M., & Miagostovich, M. (2016). Rotavirus and Astroviruses. In: J.B. Rose and B. JiménezCisneros, (eds) Global Water Pathogen Project. https://www.waterpathogens.org (J.S Meschke, and R. Girones (eds) Part 3 Viruses) https://www.waterpathogens.org/book/rotavirus Michigan State University, E. Lansing, MI, UNESCO.
Danovaro, R., Manini, E., & Dellanno, A. (2002). Higher abundance of bacteria than viruses in deep Mediterranean sediments. Applied and Environmental Microbiology, 68, 1468–1472.
Danovaro, R., & Middelboe, M. (2010). Separation of free virus particles from sediments in aquatic systems. In S. W. Wilhelm, M. G. Weinbauer, & C. A. Suttle (Eds.), Chapter 8. Manual of aquatic viral ecology, (pp. 74–81). Waco: American Society of Limnology and Oceanograph.
Dunn, G., Harris, L., Cook, C., & Prystajecky, N. A. (2014). A comparative analysis of current microbial water quality risk assessment and management practices in British Columbia and Ontario, Canada. Science of the Total Environment, 468–469, 544–552.
Eifan, S. A. (2013). Enteric viruses and aquatic environment. The Internet Journal of Microbiology, 12.
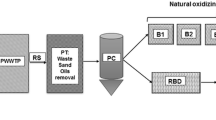
El-Fakharany, Z. (2013). Environmental impact assessment of artificial recharge of treated wastewater on groundwater aquifer system. Case study: Abu Rawash, Egypt. Journal of American Science, 9(2), 494–502.
Elhag, W. I., Saeed, H. A., Omer, E. E., & Ali, A. S. (2013). Prevalence of rotavirus and adenovirus associated with diarrhoea among displaced communities in Khartoum Sudan. BMC Infectious Diseases, 13(209), 1–6.
Elmahdy, E. M., Ahmed, N. I., Shaheen, M. N. F., Mohamed, E. B., & Loutfy, S. A. (2019). Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. Journal of Water and Health, 17, 287–294.
Elmahdy, M. E. I., Fongaro, G., Magri, M. E., Petruccio, M. M., & Barardi, C. R. (2016). Spatial distribution of enteric viruses and somatic coliphages in a lagoon used as drinking water source and recreation in Southern Brazil. International Journal of Hygiene and Environmental Health, 219, 617–625.
Fong, T. T., & Lipp, E. K. (2005). Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews, 69(2), 357–371.
Fongaro, G., Nascimento, M. A., & Viancelli, A. (2012). Surveillance of human viral contamination and physicochemical profiles in a surface water lagoon. Water Science and Technology, 66(12), 2682–2687.
Garcia, L. A. T., Viancelli, A., Rigotto, C., Pilotto, M. R., Esteves, P. A., Kunz, A., et al. (2012). Surveillance of human and swine adenovirus, human norovirus and swine circovirus in water samples in Santa Catarina, Brazil. Journal of Water and Health, 10, 445–452.
Harrach, B., Benko, M., Both, G. W., Brown, M., Davison, A. J., Echavarria, M., et al. (2012). Adenoviridae. In A. M. Q. King, M. J. Adams, E. B. Carstens & E. J. Lefkowitz (Eds.), Virus taxonomy. Classification and nomenclature of viruses ninth report of the international committee on taxonomy of viruses (pp. 125–141). San Diego: Academic Press.
He, J. W., & Jiang, S. (2005). Quantification of enterococci and human adenoviruses in environmental samples by real-time PCR. Applied and Environmental Microbiology, 71, 2250–2255.
Hernroth, B. E., Conden-Hansson, A. C., Rehnstan-Holm, A. S., et al. (2002). Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: The first Scandinavian report. Applied and Environmental Microbiology, 68, 4523–4533.
Hewitt, J., Greening, G. E., Leonard, M., & Lewis, G. D. (2013). Evaluation of human adenovirus and human polyomavirus as indicators of human sewage contamination in the aquatic environment. Water Research, 47, 6750–6761.
Huang, Z. M., Hsu, B. M., Kao, P. M., Chang, T. Y., Hsu, T. K., Ho, Y. N., et al. (2015). Prevalence, quantification, and typing of human adenoviruses detected in river water in Taiwan. Environmental Science and Pollution Research International, 22, 8359–8366.
Igor Shiklomanov, A. (1993). Igor Shiklomanov's chapter "World fresh water resources". In P. H. Gleick (Ed.), Water in crisis: A guide to the world's fresh water resources. New York: Oxford University Press.
Katayama, H., Shimasaki, A., & Ohgaki, S. (2002). Development of a virus concentration method and its application to detection of enterovirus and norwalk virus from coastal seawater. Applied and Environmental Microbiology, 68, 1033–1039.
Kern, A., Kadar, M., Szomor, K., Berencsi, G., Kapusinszky, B., & Vargha, M. (2013). Detection of enteric viruses in Hungarian surface waters: First steps towards environmental surveillance. Journal of Water and Health, 11, 772–782.
Kishida, N., Morita, H., Haramoto, E., Asami, M., & Akiba, M. (2012). One-year weekly survey of noroviruses and enteric adenoviruses in the Tone River water in Tokyo metropolitan area, Japan. Water Research, 46, 2905–2910.
Kitajima, M., Iker, B. C., Pepper, I. L., & Gerba, C. P. (2014). Relative abundance and treatment reduction of viruses during wastewater treatment processes–identification of potential viral indicators. Science of the Total Environment, 488, 290–296.
Kiulia, N. M., Hofstra, N., Vermeulen, L. C., Obara, M. A., Medema, G., & Rose, J. B. (2015). Global occurrence and emission of rotaviruses to surface waters. Pathogens, 4, 229–255.
Kiulia, N. M., Netshikweta, R., Page, N. A., Van Zyl, W. B., Kiraithe, M. M., Nyachieo, A., et al. (2010). The detection of enteric viruses in selected urban and rural river water and sewage in Kenya, with special reference to rotaviruses. Journal of Applied Microbiology, 109(3), 818–828.
La Rosa, G., Fratini, M., della Libera, S., Iaconelli, M., & Muscillo, M. (2012). Emerging and potentially emerging viruses in water environments. Annali dell’Istituto Superiore di Sanità, 48(4), 397–406.
La Rosa, G., Pourshaban, M., Iaconelli, M., & Muscillo, M. (2010). Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Annali dell'Istituto Superiore di Sanita, 46, 266–273.
Lechevallier, M. W., & Au, K. (2004). Water treatment and pathogen control process—Efficiency in achieving safe drinking water. World Health Organization (WHO) (1st ed., Vol. 1). Cornwall: TJ International.
Lewis, G. D., & Metcalf, T. G. (1988). Polyethylene glycol precipitation for recovery of pathogenic virus including hepatitis A and human rotaviruses from oysters water and sediments. Applied and Environmental Microbiology, 54, 1983–1988.
Lin, J., & Singh, A. (2015). Detection of human enteric viruses in Umgeni River, Durban, South Africa. Journal of Water and Health, 13, 1098–1112.
Lu, Q. B., Tong, Y. G., Wo, Y., Wang, H. Y., Liu, E. M., Gray, G. C., et al. (2014). Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009–2012. Influenza and Other Respiratory Viruses, 8(3), 302–308.
Mans, J., Corrie de Villiers, J., du Plessis, N. M., Avenant, T., & Taylor, M. B. (2010). Emerging norovirus GII.4 2008 variant detected in hospitalised pediatric patients in South Africa. Journal of Clinical Virology, 49(4), 258–264.
Meneses, M., Pasqualino, J., & Castells, F. (2010). Environmental assessment of urban wastewater reuse: Treatment alternatives and applications. Chemosphere, 81, 266–272.
Moresco, V., Viancelli, A., Nascimento, M. A., et al. (2012). Microbiological and physical-chemical analysis of the coastal waters of southern Brazil. Marine Pollution Bulletin, 64, 40–48.
Mostafa, M., & Peters, R. W. (2015). Manage water quality at Abu-Rawash WWTP, Egypt. Environmental Division 2015—Core programming area at the 2015 AIChE Annual Meeting (November), 335–40.
Murray, T. Y., Mans, J., & Taylor, M. B. (2013). Human calicivirus diversity in wastewater in South Africa. Journal of Applied Microbiology, 114, 1843–1853.
Nadan, S., Walter, J. E., Grabow, W. O. K., Mitchell, D. K., & Taylor, M. B. (2003). Molecular characterisation of astroviruses by reverse transcriptase PCR and sequence analysis: Comparison of clinical and environmental isolates from South Africa. Applied and Environmental Microbiology, 69(2), 747–753.
Okoh A. I., Sibanda T., & Gusha S. S. (2010). Inadequately treated wastewater as a source of human enteric viruses in the environment. International Journal of Environmental Research and Public Health, 7, 2620-2637.
Pons, W., Young, I., Truong, J., Jones-Bitton, A., McEwen, S., Pintar, K., et al. (2015). A systematic review of waterborne disease outbreaks associated with small non-community drinking water systems in Canada and the United States. PLoS ONE, 10(10), e0141646.
Prado, T., Silva, D. M., Guilayn, W. C., Rose, T. L., Gaspar, A. M., & Miagostovich, M. P. (2011). Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Research, 45, 1287–1297.
Quidort, W. L. (2013). Detection and infectivity of human adenovirus in wastewater effluent, biosolids, and shellfish, and its persistence in estuarine water. PhD Dissertation. The College of William and Mary.
Rzezutka, A., & Cook, N. (2004). Survival of human enteric viruses in the environment and food. FEMS Microbiology Reviews, 28(4), 441–453.
Schlindwein, A. D., Rigotto, C., Simões, C. M., & Barardi, C. R. (2010a). Detection of enteric viruses in sewage sludge and treated wastewater effluent. Water Science and Technology, 61, 537–544.
Schlindwein, A. D., Simões, C. M. O., & Barardi, C. R. M. (2010b). Comparative study of two extraction methods for enteric virus recovery from sewage sludge by molecular methods. Memoria’s of Institute Oswaldo Cruz, Rio de Janeiro, 104(4), 576–579.
Shaheen, M. N. F. (2017). Rotavirus gastroenteritis among hospitalized children under 5 years of age in the Middle Eastern and North African Region: A review. Eastern Mediterranean Health Journal, 25(6), 422–430.
Shaheen, M. N. F., Abd El-Daim, S. A., Ahmed, N. I., & Elmahdy, M. E. (2018). Molecular detection of three gastroenteritis viruses in in urban sewage treatment plant and river water in Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 22(5), 615–627.
Shaheen, M. N. F., Abd El-Daim, S. A., Ahmed, N. I., & Elmahdy, M. E. (2020). Environmental monitoring of Aichivirus and human Bocavirus in samples from wastewater treatment plant, drain, and River Nile in Egypt. Journal of Water and Health. https://doi.org/10.2166/wh.2019.075.
Shaheen, M. N. F., & Elmahdy, M. E. (2019a). Molecular detection of group C Rotavirus in environmental samples in Giza, Egypt. Asian Journal of Water, Environment and Pollution, 16(4), 17–22.
Shaheen, M. N. F., & Elmahdy, M. E. (2019b). Environmental monitoring of astrovirus and norovirus in Rosetta River Nile and El-Rahawy drain, Egypt. Water Supply Journal, 19(5), 1381–1387.
Shaheen, M. N. F., Elmahdy, E. M., & Chawla-Sarkar, M. (2019). Quantitative PCR-based identification of enteric viruses contaminating fresh produce and surface water used for irrigation in Egypt. Environmental Science and Pollution Research, 26(21), 21619–21628.
Sibanda, T., & Okoh, A. I. (2012). Assessment of the incidence of enteric adenovirus species and serotypes in surface water in the Eastern Cape Province of South Africa: Tyume River as a study case. The Scientific World Journal. https://doi.org/10.1100/2012/949216.
Vieira, C. B., de Abreu Corrêa, A., de Jesus, M. S., Luz, S. L., Wyn-Jones, P., Kay, D., et al. (2016). Viruses surveillance under different season scenarios of the Negro River basin, Amazonia, Brazil. Food and Environmental Virology, 8, 57–69.
Vieira, C. B., Mendes, A. C., Guimarães, F. R., Fumian, T. M., Leite, J. P., Gaspar, A. M., et al. (2012). Detection of enteric viruses in recreational waters of an urban lagoon in the city of Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz, 107, 778–784.
Wang, Y., Edward, A., McBean, E. A., & Gharabaghi, B. (2018). Increased risks of waterborne disease outbreaks in northern Ontario due to climate change. Journal of Water Management Modeling, 26, C436.
Wyer, M. D., Wyn-Jones, A. P., Kay, D., et al. (2012). Relationships between human adenoviruses and faecal indicator organisms in European recreational waters. Water Research, 46, 4130–4141.
Wyn-Jones, A. P., Carducci, A., Cook, N., et al. (2011). Surveillance of adenoviruses and noroviruses in European recreational waters. Water Research, 45, 1025–1038.
Xagoraraki, I., Yin, Z., & Svambayev, Z. (2014). Fate of viruses in water systems. Journal of Environmental Engineering, 140(7), 04014020.
Zeng, S. Q., Halkosalo, A., Salminen, M., Szakal, E. D., Puustinen, L., & Vesikari, T. (2008). One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. Virological Methods, 153, 238–240.
Zlateva, K. T., Maes, P., Rahman, M., & Ranst, M. V. (2005). Chromatography paper strip sampling of enteric adenoviruses 40 and 41 positive stool specimens. Virology Journal, 2, 6.
Acknowledgements
This work was fully funded by Academy of Scientific Research and Technology (ASRT) (2017–2019) title of the project: Improving environmental and health conditions for workers in Abu-Rawash wastewater treatment plant and remediation of pollution resources at El-Rahawy Drain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elmahdy, E.M., Shaheen, M.N.F., Rizk, N.M. et al. Quantitative Detection of Human Adenovirus and Human Rotavirus Group A in Wastewater and El-Rahawy Drainage Canal Influencing River Nile in the North of Giza, Egypt. Food Environ Virol 12, 218–225 (2020). https://doi.org/10.1007/s12560-020-09429-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-020-09429-x