Abstract
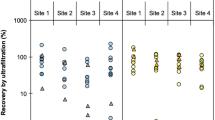
With increasing interest in peracetic acid (PAA) as a disinfectant in water treatment processes, this study determined PAA treatment effects on human noroviruses (hNoVs) genotype I (GI) and genotype II (GII) as well as effects on bacteriophage MS2 and murine norovirus (MNV) in relation to pH. Across all pH conditions, PAA achieved between 0.2 and 2.5 log10 reduction of hNoVs over 120 min contact time in buffer solution as measured by reverse transcription-qPCR (RT-qPCR). The PAA treatments produced similar RT-qPCR reductions of MS2 and MNV, in the range of 0.2–2.7 log10. Infectivity assays achieved > 4 log10 reduction of both MS2 and MNV in buffer solution after 120 min contact time. Comparing PAA activity across varying pH, disinfection at pH 8.5, in general, resulted in less reduction of infectivity and molecular signals compared to pH conditions of 6.5 and 7.5. This difference was most pronounced for reductions in infectivity of MNV and MS2, with as much as 2.7 log10 less reduction at pH 8.5 relative to lower pH conditions. This study revealed that PAA was an effective disinfectant for treatment of hNoV GI and GII, MS2 and MNV, with greatest virus reduction observed for MS2 and MNV infectivity. RT-qPCR reductions of MS2 and MNV were lower than concurrent MS2 and MNV infectivity reductions, suggesting that observed hNoV RT-qPCR reductions may underestimate reductions in hNoV infectivity achieved by PAA. Although virus disinfection by PAA occurred at all evaluated pH levels, PAA is most effective at pH 6.5–7.5.






Similar content being viewed by others
References
Ahmed, S. M., Hall, A. J., Robinson, A. E., Verhoef, L., Premkumar, P., Parashar, U. D., et al. (2014). Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infectious Diseases, 14(8), 725–730. https://doi.org/10.1016/s1473-3099(14)70767-4.
Anotai, J. (1996). Effect of calcium ion on chemistry and disinfection efficiency of free chlorine at pH 10. Drexel University.
Bae, J., & Schwab, K. J. (2008). Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Applied and Environmental Microbiology, 74(2), 477–484. https://doi.org/10.1128/aem.02095-06.
Baldry, M. G. C., & French, M. S. (1989). Activity of peracetic-acid against sewage indicators organisms. Water Science and Technology, 21(12), 1747–1749.
Baldry, M. G. C., French, M. S., & Slater, D. (1991). The activity of peracetic-acid on sewage indicator bacteria and viruses. Water Science and Technology, 24(2), 353–357.
Briancesco, R., Veschetti, E., Ottaviani, M., & Bonadonna, L. (2005). Peracetic acid and sodium hypochlorite effectiveness in reducing resistant stages of microorganisms. Central European Journal of Public Health, 13(3), 159.
Butot, S., Putallaz, T., & Sanchez, G. (2008). Effects of sanitation, freezing and frozen storage on enteric viruses in berries and herbs. International Journal of Food Microbiology, 126(1–2), 30–35. https://doi.org/10.1016/j.ijfoodmicro.2008.04.033.
Cowman, G. A., & Singer, P. C. (1996). Effect of bromide ion on haloacetic acid speciation resulting from chlorination and chloramination of aquatic humic substances. Environmental Science & Technology, 30(1), 16–24. https://doi.org/10.1021/es9406905.
Crebelli, R., Conti, L., Monarca, S., Feretti, D., Zerbini, I., Zani, C., et al. (2005). Genotoxicity of the disinfection by-products resulting from peracetic acid- or hypochlorite-disinfected sewage wastewater. Water Research, 39(6), 1105–1113. https://doi.org/10.1016/j.watres.2004.12.029.
Cromeans, T., Park, G. W., Costantini, V., Lee, D., Wang, Q., Farkas, T., et al. (2014). Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Applied and Environmental Microbiology, 80(18), 5743–5751. https://doi.org/10.1128/aem.01532-14.
Dunkin, N., Weng, S. C., Coulter, C. G., Jacangelo, J. G., & Schwab, K. J. (2017a). Reduction of human norovirus GI, GII, and surrogates by peracetic acid and monochloramine in municipal secondary wastewater effluent. Environmental Science & Technology. https://doi.org/10.1021/acs.est.7b02954.
Dunkin, N., Weng, S. C., Schwab, K. J., McQuarrie, J., Bell, K., & Jacangelo, J. G. (2017b). Comparative inactivation of murine norovirus and MS2 bacteriophage by peracetic acid and monochloramine in municipal secondary wastewater effluent. Environmental Science & Technology, 51(5), 2972–2981. https://doi.org/10.1021/acs.est.6b05529.
Ettayebi, K., Crawford, S. E., Murakami, K., Broughman, J. R., Karandikar, U., Tenge, V. R., et al. (2016). Replication of human noroviruses in stem cell-derived human enteroids. Science, 353(6306), 1387–1393. https://doi.org/10.1126/science.aaf5211.
Fraisse, A., Temmam, S., Deboosere, N., Guillier, L., Delobel, A., Maris, P., et al. (2011). Comparison of chlorine and peroxyacetic-based disinfectant to inactivate Feline calicivirus, Murine norovirus and hepatitis A virus on lettuce. International Journal of Food Microbiology, 151(1), 98–104. https://doi.org/10.1016/j.ijfoodmicro.2011.08.011.
Gibson, K. E., Opryszko, M. C., Schissler, J. T., Guo, Y. Y., & Schwab, K. J. (2011). Evaluation of human enteric viruses in surface water and drinking water resources in southern Ghana. American Journal of Tropical Medicine and Hygiene, 84(1), 20–29. https://doi.org/10.4269/ajtmh.2011.10-0389.
Haas, C. N., & Joffe, J. (1994). Disinfection under dynamic conditions—modification of hom model for decay. Environmental Science & Technology, 28(7), 1367–1369. https://doi.org/10.1021/es00056a028.
Haas, C. N., Joffe, J., Anmangandla, U., Hornberger, J., Heath, M., & Glicker, J. (1995). Development and validation of rational design methods of disinfection. AWWAR.
Hewitt, J., Rivera-Aban, M., & Greening, G. E. (2009). Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. Journal of Applied Microbiology, 107(1), 65–71. https://doi.org/10.1111/j.1365-2672.2009.04179.x.
Hwang, S., Alhatlani, B., Arias, A., Caddy, S. L., Christodoulou, C., Cunha, J. B., et al. (2014). Murine norovirus: Propagation, quantification, and genetic manipulation. Current Protocols in Microbiology, 33, 15k. https://doi.org/10.1002/9780471729259.mc15k02s33.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., Hoshino, F. B., et al. (2003). Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41(4), 1548–1557. https://doi.org/10.1128/jcm.41.4.1548-1557.2003.
Kitajima, M., Tohya, Y., Matsubara, K., Haramoto, E., Utagawa, E., & Katayama, H. (2010). Chlorine inactivation of Human norovirus, murine norovirus and poliovirus in drinking water. Letters in Applied Microbiology, 51(1), 119–121. https://doi.org/10.1111/j.1472-765X.2010.02869.x.
Kitis, M. (2004). Disinfection of wastewater with peracetic acid: A review. Environment International, 30(1), 47–55. https://doi.org/10.1016/s0160-4120(03)00147-8.
Knight, A., Haines, J., Stals, A., Li, D., Uyttendaele, M., Knight, A., et al. (2016). A systematic review of human norovirus survival reveals a greater persistence of human norovirus RT-qPCR signals compared to those of cultivable surrogate viruses. International Journal of Food Microbiology, 216, 40–49. https://doi.org/10.1016/j.ijfoodmicro.2015.08.015.
Lopman, B. A., Hall, A. J., Curns, A. T., & Parashar, U. D. (2011). Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clinical Infectious Diseases, 52(4), 466–474. https://doi.org/10.1093/cid/ciq163.
Luukkonen, T., & Pehkonen, S. O. (2017). Peracids in water treatment: A critical review. Critical Reviews in Environmental Science and Technology, 47(1), 1–39.
Mandal, H. K. (2014). Influence of wastewater pH on turbidity. International Journal of Environmental Research and Development, 4, 105–114.
Mayer, B. K., Ryu, H., & Abbaszadegan, M. (2008). Treatability of U.S. Environmental Protection Agency contaminant candidate list viruses: Removal of coxsackievirus and echovirus using enhanced coagulation. Environmental Science & Technology, 42(18), 6890–6896. https://doi.org/10.1021/es801481s.
Mayer, B. K., Yang, Y., Gerrity, D. W., & Abbaszadegan, M. (2015). The impact of capsid proteins on virus removal and inactivation during water treatment processes. Microbiology Insights, 8, MBI-S31441.
McFadden, M., Loconsole, J., Schockling, A., Nerenberg, R., & Pavissich, J. P. (2017). Comparing peracetic acid and hypochlorite for disinfection of combined sewer overflows: Effects of suspended-solids and pH. Science of the Total Environment, 599, 533–539. https://doi.org/10.1016/j.scitotenv.2017.04.179.
Mukherjee, S. K., Chatterji, A. K., & Saraswat, I. P. (1968). Effect of pH on the rate of BOD of wastewater. Journal (Water Pollution Control Federation), 40(11), 1934–1939.
Park, G. W., Boston, D. M., Kase, J. A., Sampson, M. N., & Sobsey, M. D. (2007). Evaluation of liquid- and fog-based application of sterilox hypochlorous acid solution for surface inactivation of human norovirus. Applied and Environmental Microbiology, 73(14), 4463–4468. https://doi.org/10.1128/aem.02839-06.
Richards, G. P. (2012). Critical review of norovirus surrogates in food safety research: Rationale for considering volunteer studies. Food and Environmental Virology, 4(1), 6–13. https://doi.org/10.1007/s12560-011-9072-7.
Sagripanti, J. L., & Bonifacino, A. (1996). Comparative sporicidal effects of liquid chemical agents. Applied and Environmental Microbiology, 62(2), 545–551.
SanchezRuiz, C., MartinezRoyano, S., & TejeroMonzon, I. (1995). An evaluation of the efficiency and impact of raw wastewater disinfection with peracetic acid prior to ocean discharge. Water Science and Technology, 32(7), 159–166. https://doi.org/10.1016/0273-1223(96)00060-1.
Springthorpe, S., & Sattar, S. A. (2007). Chapter 6 Virus removal during drinking water treatment. In A. Bosch (Ed.), Perspectives in medical virology (Vol. 17, pp. 109–126). Amsterdam: Elsevier.
Tung, G., Macinga, D., Arbogast, J., & Jaykus, L. A. (2013). Efficacy of commonly used disinfectants for inactivation of human noroviruses and their surrogates. Journal of Food Protection, 76(7), 1210–1217. https://doi.org/10.4315/0362-028x.jfp-12-532.
USEPA. (2001). Method 1602: Male-specific (F+) and somatic coliphage in water by single agar layer (SAL) procedure. Washington, D. C.: U.S. Environmental Protection Agency.
Veschetti, E., Cutilli, D., Bonadonna, L., Briancesco, R., Martini, C., Cecchini, G., et al. (2003). Pilot-plant comparative study of peracetic acid and sodium hypochlorite wastewater disinfection. Water Research, 37(1), 78–94. https://doi.org/10.1016/s0043-1354(02)00248-8.
Watson, H. E. (1908). A note on the variation of the rate of disinfection with change in the concentration of the disinfectant. Journal of Hygiene, 8(4), 536–542.
Watson, K., Shaw, G., Leusch, F. D. L., & Knight, N. L. (2012). Chlorine disinfection by-products in wastewater effluent: Bioassay-based assessment of toxicological impact. Water Research, 46(18), 6069–6083. https://doi.org/10.1016/j.watres.2012.08.026.
Wigginton, K. R., Pecson, B. M., Sigstam, T., Bosshard, F., & Kohn, T. (2012). Virus inactivation mechanisms: Impact of disinfectants on virus function and structural integrity. Environmental Science & Technology, 46(21), 12069–12078. https://doi.org/10.1021/es3029473.
Zonta, W., Mauroy, A., Farnir, F., & Thiry, E. (2016). Comparative virucidal efficacy of seven disinfectants against murine norovirus and Feline calicivirus, surrogates of human norovirus. Food and Environmental Virology, 8(1), 1–12. https://doi.org/10.1007/s12560-015-9216-2.
Acknowledgements
This work was supported by the National Science Foundation Graduate Research Fellowship Program (Award ID# DGE-1232825), the JHU/Stantec Alliance, the Johns Hopkins Water Institute (Award ID GW-0010), and the Osprey Foundation (Award ID OFM-0014) of Maryland. We thank Dr. Charles Haas for insightful discussions regarding modeling of hNoV and viral surrogates and PeroxyChem Inc. for providing PAA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dunkin, N., Coulter, C., Weng, S. et al. Effects of pH Variability on Peracetic Acid Reduction of Human Norovirus GI, GII RNA, and Infectivity Plus RNA Reduction of Selected Surrogates. Food Environ Virol 11, 76–89 (2019). https://doi.org/10.1007/s12560-018-9359-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-018-9359-z