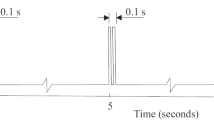
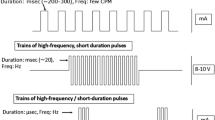
Abstract
Gastric electrical stimulation is a minimally invasive and alternative treatment modality for patients suffering from motility disorders and obesity. The idea of inducing motility and correcting dysrhythmia by the application of an appropriate stimulation (also referred to as electrical stimulation) has gained interest among the clinicians. Injecting current at the acupuncture points via needle (electroacupuncture) or surface electrodes (transcutaneous electroacupuncture) is an emerging field of study for motility disorders. With reference to the clinical studies, the usefulness of the method is considered for their efficacy in symptomatic control and disease management. Although electrical stimulation is shown be a promising technology in the recent years, their use as a treatment option is not encouraging since the mechanism of action and efficacy on reducing symptoms is not clear. A multilateral study involving theoretical modelling and computer simulation proves useful in understanding the mechanisms involved in the therapeutics. In this article, we review the electrical stimulation as an alternative approach for management of the patients suffering from motility dysfunctions and obesity.
Similar content being viewed by others
Availability of data and material
(data transparency). None.
Code availability
(software application or custom code). None.
References
Kelly KA, Code CF. Canine gastric pacemaker. Am J Physiol. 1971;220(1):112–8.
Weber Jr J, Koatsu S. Pacemaker localization and electrical conduction patterns in the canine stomach. Gastroenterology. 1970 Nov 1;59(5):717–26.
Kelly KA, La Force RC. Pacing the canine stomach with electric stimulation. Am J Physiol. 1972;222(3):588–94.
Daniel EE, Chapman KM. Electrical activity of the gastrointestinal tract as an indication of mechanical activity The American journal of digestive diseases. 1963;8(1):54–102.
Kelly KA, La Force RC. Role of the gastric pacesetter potential defined by electrical pacing. Can J Physiol Pharmacol. 1972;50(10):1017–9.
Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surg. 1977;133(1): p. 29–33.
Lin ZY, et al. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998;274(1):G186–91.
Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006;24(7):991–1002.
McCallum RW, et al. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114(3):456–61.
Hasler WL. Methods of gastric electrical stimulation and pacing: a review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil. 2009;21(3):229–43.
Avvari RK. Managing Motility Disorders of the Antro-Pyloro-Duodenal Segment: A Biomedical Engineering Perspective. United Journal of Biochemistry and Biotechnology. 2019;1(2):1–20.
Avvari RK. Bio-mechanics of the distal stomach and duodenum: An insight into mechanisms of duodenogastric reflux and duodenal mixing, in Department of Biological Sciences and Bioengineering. 48th Graduating Students Convocation, Indian Institute of Technology Kanpur. 2015.
Avvari RK. Enteric and Central Nervous System Mediated Control of Digestive Processes in the Small Intestine: a Coprocessor-Processor Paradigm. Food Science and Engineering. 2020;1(1):37–42.
Fox M, Avvari RK, Kaufman E. The mechanism of reflux suppression by alginates visualized by magnetic resonance imaging and manometry. in Digestive Disease Week. 2011.
Sweis R, et al. Post-prandial reflux suppression by a raft-forming alginate (Gaviscon Advance) compared to a simple antacid documented by magnetic resonance imaging and pH-impedance monitoring: mechanistic assessment in healthy volunteers and randomised, controlled, double-blind study in reflux patients. Aliment Pharmacol Ther. 2013;37(11):1093–102.
Lal N, et al. Gastric Electrical Stimulation with the Enterra System: A Systematic Review. Gastroenterol Res Pract. 2015;762972.
Al-Shboul OA. The importance of interstitial cells of cajal in the gastrointestinal tract. Saudi J Gastroenterol. 2013;19(1):3–15.
Mazzone A, Farrugia G. Evolving concepts in the cellular control of gastrointestinal motility: neurogastroenterology and enteric sciences. Gastroenterol Clin North Am. 2007;36(3):499–513, vii.
Beckett EA, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neuro. 2005;493(2):193–206.
O’Grady G, et al. Origin and propagation of human gastric slow-wave activity defined by high-resolution mapping. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G585–92.
Christensen J, Schedl HP, Clifton JA. The small intestinal basic electrical rhythm (slow wave) frequency gradient in normal men and in patients with variety of diseases. Gastroenterology. 1966;50(3):309–15.
Rinecker H, Chaussy C, Brendel W. The propagation of contractile waves from duodenum to jejunum. Pflugers Arch. 1969;305(3):210–8.
Milton GW, Smith AW. The pacemaking area of the duodenum. J Physiol. 1956;132(1):100–14.
Hermon-Taylor J, Code CF. Localization of the duodenal pacemaker and its role in the organization of duodenal myoelectric activity. Gut. 1971;12(1):40–7.
Huizinga JD, Chen JH. The myogenic and neurogenic components of the rhythmic segmentation motor patterns of the intestine. Front Neurosci. 2014;8:78.
Lammers WJ, et al. Multielectrode mapping of slow-wave activity in the isolated rabbit duodenum. J Appl Physiol. 1993;74(3):1454–61.
Egbuji JU, et al. Origin, propagation and regional characteristics of porcine gastric slow wave activity determined by high-resolution mapping. Neurogastroenterol Motil. 2010;22(10):e292-300.
Cheng LK, Du P, O’Grady G. Mapping and modeling gastrointestinal bioelectricity: from engineering bench to bedside. Physiology (Bethesda). 2013;28(5):310–7.
O’Grady G, et al. Rapid high-amplitude circumferential slow wave propagation during normal gastric pacemaking and dysrhythmias. Neurogastroenterol Motil. 2012;24(7):e299-312.
O'Grady G, et al. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143(3):589–598 e3.
O’Grady G, et al. Recent progress in gastric arrhythmia: pathophysiology, clinical significance and future horizons. Clin Exp Pharmacol Physiol. 2014;41(10):854–62.
Lammers WJ. Inhomogeneities in the propagation of the slow wave in the stomach. Neurogastroenterol Motil. 2015;27(10):1349–53.
Du P, et al. The impact of surgical excisions on human gastric slow wave conduction, defined by high-resolution electrical mapping and in silico modeling. Neurogastroenterol Motil. 2015;27(10):1409–22.
Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296(1):G1-8.
O’Grady G, Abell TL. Gastric arrhythmias in gastroparesis: low- and high-resolution mapping of gastric electrical activity. Gastroenterol Clin North Am. 2015;44(1):169–84.
Pal A, et al. Gastric flow and mixing studied using computer simulation. Proc R Soc of Lond B. 2004;271(1557):2587–94.
Ravi Kant A. Bio-mechanics of the distal stomach and duodenum: An insight into mechanisms of duodenogastric reflux and duodenal mixing, in Bio Sci Bioeng. IIT Kanpur: Kanpur. 2015.
Nguyen HN, et al. Postprandial transduodenal bolus transport is regulated by complex peristaltic sequence. World J Gastroenterol. 2006;12(37):6008–16.
Lammers WJ. Arrhythmias in the gut. Neurogastroenterol Motil. 2015;25(5):353–7.
Maranki J, Parkman HP. Gastric electric stimulation for the treatment of gastroparesis. Curr Gastroenterol Rep. 2007;9(4):286–94.
Xing J, et al. The effect of gastric electrical stimulation on canine gastric slow waves. Am J Physiol Gastrointest Liver Physiol. 2003;284(6):G956–62.
Eagon JC, Kelly KA. Effects of gastric pacing on canine gastric motility and emptying. Am J Physiol. 1993;265(4 Pt 1):G767–74.
Familoni BO, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42(5):892–7.
Salem A, Gaber O. Electrical stimulation at a frequency higher than basal rate in human stomach. Digestive diseases and sciences. 1997;42(5).
Horn CC, Ardell JL, Fisher LE. Electroceutical Targeting of the Autonomic Nervous System. Physiology (Bethesda). 2019;34(2):150–62.
Payne SC, Furness JB, Stebbing MJ. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat Rev Gastroenterol Hepatol. 2019;16(2):89–105.
Andrews PL, et al. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990;68(2):325–45.
Val-Laillet D, et al. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs. Appetite. 2010;55(2):245–52.
McCallum RW, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25(10):815-e636.
Moore JS, Gibson PR, Burgell RE. Neuromodulation via Interferential Electrical Stimulation as a Novel Therapy in Gastrointestinal Motility Disorders. J Neurogastroenterol Motil. 2018;24(1):19–29.
Voll R, Twenty years of electroacupuncture diagnosis in Germany. A progress report. 1975.
Chen XH, Han JS. All three types of opioid receptors in the spinal cord are important for 2/15 Hz electroacupuncture analgesia. Eur J Pharma. 1992;211(2):203–210.
Han JS, et al. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain. 1991;47(3):295–8.
Abell T, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125(2):421–8.
Lin Z, et al. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27(5):1071–6.
Mason RJ,et al. Gastric electrical stimulation: an alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140(9): 841–6; discussion 847–8.
Lin Z, et al. Symptom responses, long-term outcomes and adverse events beyond 3 years of high-frequency gastric electrical stimulation for gastroparesis. Neurogastroenterol Motil. 2006;18(1):18–27.
Brody F, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207(4):533–8.
McCallum RW, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol, 2010;8(11):947–54; quiz e116.
McCallum RW, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9(4):314–319 e1.
Bohdjalian A, et al. One-year experience with Tantalus: a new surgical approach to treat morbid obesity. Obes Surg. 2006;16(5):627–34.
McKenzie PSA, Du P, Lahr C, Cheng LK, McElmurray L, Kedar A, Boatright B, Hassan H, Hughes M, Omer E. The effect of gastric electrical stimulation on small bowel motility in patients with gastroparesis and concomitant pancreatic and small bowel dysfunction: From animal model to human application. Neuromodulation: Techno at the Neural Inter. 2019;22(6):723–9.
Zeng Y, et al. Efficacy of electroacupuncture compared with transcutaneous electric nerve stimulation for functional constipation: Study protocol for a randomized, controlled trial. Medicine. 2018;97(19).
Yik YI, Hutson J, Southwell B. Home-based transabdominal interferential electrical stimulation for six months improves paediatric slow transit constipation (STC). Neuromodulation. 2018;21(7):676–81.
Leong LC, et al. Long-term effects of transabdominal electrical stimulation in treating children with slow-transit constipation. J Pediatr Surg. 2011;46(12):2309–12.
Dinning PG, et al. Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two-phase, double-blind randomized controlled crossover study. Am J Gastroenterol. 2015;110(5):733–40.
Yiannakou Y, et al. A randomized double-blinded sham-controlled cross-over trial of tined-lead sacral nerve stimulation testing for chronic constipation. Eur J Gastroenterol Hepatol. 2019;31(6):653–60.
Moore JS, Gibson PR, Burgell RE. Randomised clinical trial: transabdominal interferential electrical stimulation vs sham stimulation in women with functional constipation. Aliment Pharmacol Ther. 2020;51(8):760–9.
Shi N, et al. Transcutaneous electrical nerve stimulation improves oppilative symptoms and increases colonic transit in patients with slow transit constipation. Zhonghua Yi Xue Za Zhi. 2009;89(14):947–50.
Besendorfer M, et al. A Pilot Study of Non-invasive Sacral Nerve Stimulation in Treatment of Constipation in Childhood and Adolescence. Front Pediatr. 2020;8:169.
Clarke MC, et al. Decreased colonic transit time after transcutaneous interferential electrical stimulation in children with slow transit constipation. J Pediatr Surg. 2009;44(2):408–12.
Patton V, et al. Sacral Nerve Stimulation Fails to Offer Long-term Benefit in Patients With Slow-Transit Constipation. Dis Colon Rectum. 2016;59(9):878–85.
Leroi AM, et al. Transcutaneous electrical tibial nerve stimulation in the treatment of fecal incontinence: a randomized trial (CONSORT 1a). Am J Gastroenterol. 2012;107(12):1888–96.
Thin NN, et al. Randomized clinical trial of sacral versus percutaneous tibial nerve stimulation in patients with faecal incontinence. Br J Surg. 2015;102(4):349–58.
Tjandra JJ, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
van der Wilt AA, et al. Randomized clinical trial of percutaneous tibial nerve stimulation versus sham electrical stimulation in patients with faecal incontinence. Br J Surg. 2017;104(9):1167–76.
Horrocks EJ, et al. Double-blind randomised controlled trial of percutaneous tibial nerve stimulation versus sham electrical stimulation in the treatment of faecal incontinence: CONtrol of Faecal Incontinence using Distal NeuromodulaTion (the CONFIDeNT trial). Health Technol Assess. 2015;19(77):1–164.
Sanmiguel CP, et al. The TANTALUS system for obesity: effect on gastric emptying of solids and ghrelin plasma levels. Obes Surg. 2007;17(11):1503–9.
Alarcon Del Agua I, et al. Post-implant Analysis of Epidemiologic and Eating Behavior Data Related to Weight Loss Effectiveness in Obese Patients Treated with Gastric Electrical Stimulation. Obes Surg. 2017;27(6):1573–80.
Horbach T, et al. Closed-loop gastric electrical stimulation versus laparoscopic adjustable gastric band for the treatment of obesity: a randomized 12-month multicenter study. Int J Obes (Lond). 2016;40(12):1891–8.
Shikora SA, et al. Implantable gastric stimulation for the treatment of clinically severe obesity: results of the SHAPE trial. Surg Obes Relat Dis. 2009;5(1):31–7.
Horbach T, et al. abiliti® closed-loop gastric electrical stimulation system for treatment of obesity: clinical results with a 27-month follow-up. Obes Surg. 2015;25(10):1779–87.
Abell TL, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66(4):204–12.
Huizinga JD, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373(6512):347–9.
Wei R, Parsons SP, Huizinga JD. Network properties of interstitial cells of Cajal affect intestinal pacemaker activity and motor patterns, according to a mathematical model of weakly coupled oscillators. Exp Physiol. 2017;102(3):329–46.
Cheng LK, et al. Strategies to refine gastric stimulation and pacing protocols: experimental and modeling approaches. Front Neurosci. 2021;15:645472.
Pullan A, et al. Modelling gastrointestinal bioelectric activity. Prog Biophys Mol Biol. 2004;85(2–3):523–50.
Jarrett ME, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91(12):1559–69.
Koklu S, et al. Clinical trial: interferential electric stimulation in functional dyspepsia patients - a prospective randomized study. Aliment Pharmacol Ther. 2010;31(9):961–8.
Coban S, et al. Clinical trial: transcutaneous interferential electrical stimulation in individuals with irritable bowel syndrome - a prospective double-blind randomized study. Digestion. 2012;86(2):86–93.
Gourcerol G, et al. How sacral nerve stimulation works in patients with faecal incontinence. Colorectal Dis. 2011;13(8):e203–11.
Yousif N, Vaizey CJ, Maeda Y. Mapping the current flow in sacral nerve stimulation using computational modelling. Healthcare technology letters. 2019;6(1):8–12.
Zhang WB. et al. Effects and mechanism of acupuncture based on the principle of meridians. Evid Based Complement Alternat Med. 2013;684027.
Zhou W, Benharash P. Effects and mechanisms of acupuncture based on the principle of meridians. J Acupunct Meridian Stud. 2014;7(4):190–3.
Hu S. et al. Electroacupuncture at Zusanli (ST36) Prevents intestinal barrier and remote organ dysfunction following gut ischemia through activating the cholinergic anti-inflammatory-dependent mechanism. Evid Based Complement Alternat Med. 2013;592127.
Shuai X, et al. Different effects of electroacupuncture on esophageal motility and serum hormones in cats with esophagitis. Dis Esophagus. 2008;21(2):170–5.
Chang CS, et al. Cutaneous electrical stimulation of acupuncture points may enhance gastric myoelectrical regularity. Digestion. 2002;66(2):106–11.
Stein B, Everhart KK, Lacy BE. Gastroparesis: A Review of Current Diagnosis and Treatment Options. J Clin Gastroenterol. 2015;49(7):550–8.
Enweluzo C, Aziz F. Gastroparesis: a review of current and emerging treatment options. Clin Exp Gastroenterol. 2013;6:161–5.
Quigley EMM. Prokinetics in the Management of Functional Gastrointestinal Disorders. Curr Gastroenterol Rep. 2015;19(10):53.
Roe NA, et al. Evaluation of prokinetic agents used in the treatment of gastroparesis. J Drug Assess. 2017;6(1):6–9.
Homko CJ, et al. Effect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2015;27(4):501–8.
Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr. 2016;55(3):897–906.
Chen JD, Yin J, Wei W. Electrical therapies for gastrointestinal motility disorders. Expert Rev Gastroenterol Hepatol. 2017;11(5):407–18.
Soffer E, et al. Review article: gastric electrical stimulation for gastroparesis–physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30(7):681–94.
Camilleri M, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37; quiz 38.
Haba T, Sarna SK. Regulation of gastroduodenal emptying of solids by gastropyloroduodenal contractions. Am J Physiol. 1993;264(2 Pt 1):G261–71.
Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90(6):1919–25.
Miller LS, et al. Treatment of idiopathic gastroparesis with injection of botulinum toxin into the pyloric sphincter muscle. Am J Gastroenterol. 2002;97(7):1653–60.
Tack J, et al. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. 2009;6(10):583–90.
Acosta A, Camilleri M. Prokinetics in gastroparesis. Gastroenterol Clin North Am. 2015;44(1):97–111.
Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98(2):259–63.
Nasr I, et al. Effects of tegaserod and erythromycin in upper gut dysmotility: a comparative study. Indian J Gastroenterol. 2009;28(4):136–42.
Berthet S, Charpiat B, Mabrut JY. Erythromycin as a prokinetic agent: Risk factors. J Visc Surg. 2010;147:e13–8.
Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31(1):11–9.
Morris AD, et al. Domperidone-Associated QT Interval prolongation in non-oncologic pediatric patients: A review of the literature. Can J Hosp Pharm. 2016;69(3):224–30.
Makari J, Cameron K, Battistella M. Domperidone-associated sudden cardiac death in the general population and implications for use in patients undergoing hemodialysis: a literature review. Can J Hosp Pharm. 2014;67(6):441–6.
Rossi M, Giorgi G. Domperidone and long QT syndrome. Curr Drug Saf. 2010;5(3):257–62.
van Zanten AR, et al. Still a Place for Metoclopramide as a Prokinetic Drug in Critically Ill Patients? JPEN J Parenter Enteral Nutr. 2015;39(7):763–6.
Scarpignato C. Pharmacological stimulation of gastrointestinal motility: where we are and where are we going? Dig Dis. 1997;15(Suppl 1):112–36.
Ohno T, Mochiki E, Kuwano H. The roles of motilin and ghrelin in gastrointestinal motility. Int J Pept. 2010.
Zala AV, Walker MM, Talley NJ. Emerging drugs for functional dyspepsia. Expert Opin Emerg Drugs. 2015;20(2):221–33.
Tack J, Janssen P. Emerging drugs for functional dyspepsia. Expert Opin Emerg Drugs. 2011;16(2):283–92.
Sanger GJ, Furness JB. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2015;13(1):38–48.
Chedid V, Camilleri M. Relamorelin for the treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs. 2017;26(10):1189–97.
Cha R, Marescaux J, Diana M. Updates on gastric electrical stimulation to treat obesity: Systematic review and future perspectives. World J Gastrointest Endosc. 2014;6(9):419–31.
Chiu JD, Soffer E. Gastric electrical stimulation for obesity. Curr Gastroenterol Rep. 2015;17(1):424.
Mintchev MP. Gastric electrical stimulation for the treatment of obesity: from entrainment to bezoars-a functional review. ISRN Gastroenterol. 2013;434706.
Brody F, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220(1):57–63.
Wo JM, et al. Gastric Electrical Stimulation for Gastroparesis and Chronic Unexplained Nausea and Vomiting. Curr Treat Options Gastroenterol. 2016;14(4):386–400.
Islam S, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51(1):67–71.
Teich S. Electrical stimulation of the GI Tract. Pediatric Neurogastro. 2016;499–505.
Lebovitz HE. Interventional treatment of obesity and diabetes: An interim report on gastric electrical stimulation. Rev Endocr Metab Disord. 2016;17(1):73–80.
Gonzalez HC, Velanovich V. Enterra Therapy: gastric neurostimulator for gastroparesis. Expert Rev Med Devices. 2010;7(3):319–32.
Lin Z, Chen JD. Advances in gastrointestinal electrical stimulation. Crit Rev Biomed Eng. 2002;30(4–6):419–57.
Liu S, Hou X, Chen JD. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterol. 2005;100(4):792–6.
Aberle J, et al. Duodenal Electric Stimulation: Results of a First-in-Man Study. Obes Surg. 2016;26(2):369–75.
Xu X, Zhu H, Chen JD. Pyloric electrical stimulation reduces food intake by inhibiting gastric motility in dogs. Gastroenterology. 2005;128(1):43–50.
Ray K. Therapy: Gastric electrical stimulation relieves nausea and vomiting in the long term. Nat Rev Gastroenterol Hepatol. 2012;9(5):243.
Gourcerol G, et al. Gastric electrical stimulation in medically refractory nausea and vomiting. Eur J Gastroenterol Hepatol. 2007;19(1):29–35.
Yin J, et al. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation. 2012;15(3):224–31; discussion 231.
Kong MF, et al. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22(3):503–7.
Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43.
Klein S, et al. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun. 2013;4:1630.
Blair PJ, et al. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20(3):294–317.
Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999;75(1):38–50.
Takaki M. Gut pacemaker cells: the interstitial cells of Cajal (ICC). J Smooth Muscle Res. 2003;39(5):137–61.
Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl 1):54–63.
Bashashati M, McCallum RW. Is Interstitial Cells of Cajalopathy Present in Gastroparesis? J Neurogastroenterol Motil. 2015;21(4):486–93.
Zhang J, Chen JD. Pacing the gut in motility disorders. Curr Treat Options Gastroenterol. 2006;9(4):351–60.
Song GQ, et al. Gastric electrical stimulation with long pulses in humans and animals: can data obtained in animals be replicated in humans? Neuromodulation. 2010;13(2):87–92.
Li S, Chen JD. Pulse Width-Dependent Effects of Intestinal Electrical Stimulation for Obesity: Role of Gastrointestinal Motility and Hormones. Obes Surg. 2017;27(1):70–7.
Du P, et al. A multiscale model of the electrophysiological basis of the human electrogastrogram. Biophys J. 2010;99(9):2784–92.
Corrias A, et al. Modelling tissue electrophysiology with multiple cell types: applications of the extended bidomain framework. Integr Biol (Camb). 2012;4(2):192–201.
Pauwels A, et al. Gastric emptying and different types of reflux in adult patients with cystic fibrosis. Aliment Pharmacol Ther. 2011;34(7):799–807.
Hausken T, et al. Quantification of gastric emptying and duodenogastric reflux stroke volumes using three-dimensional guided digital color Doppler imaging. Eur J Ultrasound. 2001;13(3):205–13.
Hausken T, et al. Antroduodenal motility and movements of luminal contents studied by duplex sonography. Gastroenterology. 1992;102(5):1583–90.
Dillard S, Krishnan S, Udaykumar HS. Mechanics of flow and mixing at antroduodenal junction. World J Gastroenterol. 2007;13(9):1365–71.
Brasseur JG. A fluid mechanical perspective on esophageal bolus transport. Dysphagia. 1987;2(1):32–9.
Brasseur JG, et al. Function of longitudinal vs circular muscle fibers in esophageal peristalsis, deduced with mathematical modeling. World J Gastroenterol. 2007;13(9):1335–46.
Pal A. Motility of the pharynx analyzed using lattice Boltzmann simulation, in Mech Eng. Pennsylvania State University: University Park. 2000;186.
Pal A, et al. Application of a virtual stomach to evaluate gastric mixing and breakdown of solid food. Gastroenterology. 2003;124(4):A673–4.
Pal A, Brasseur JG. The mechanical advantage of local longitudinal shortening on peristaltic transport. J Biomech Eng. 2002;124(1):94–100.
Pal A, Brasseur JG, Abrahamsson B. A stomach road or “Magenstrasse” for gastric emptying. J Biomech. 2007;40(6):1202–10.
Pal A, et al. Pressure-geometry relationships in the stomach analyzed through computer stimulation. Gastroenterology. 1999;116(4):A1057–A1057.
Pal A, Verma D. Local longitudinal muscle contraction of the stomach during gastric peristalsis in Wistar rats (abstract). Neurogastroenterol and Motility. 2008;20(Suppl 2):47.
Pal A, et al. Intrabolus pressure gradient identifies pathological constriction in the upper esophageal sphincter during flow. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G1037–48.
Pal A, et al. Application of computer simulation to the evaluation of pathophysiology of the pharyngo-esophageal (PE) segment. Gastroenterology. 2001;120(5):A221–A221.
Faas H, et al. Pressure-geometry relationship in the antroduodenal region in humans. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1214–20.
Indireshkumar K, et al. Relative contributions of "pressure pump" and "peristaltic pump" to gastric emptying. Am J of Physiol Gastrointest Liver Physiol. 2000;278(4):G604-G616.
Pallotta N, et al. Antro-pyloric contractile patterns and transpyloric flow after meal ingestion in humans. Am J Gastroenterol. 1998;93(12):2513–22.
Pallotta N, et al. Antro-pyloro-duodenal common chamber (APDCC) and antral contractions in the regulation of gastric emptying of liquid and solid meals. Gastroenterology. 1996;110(4):A728–A728.
Pallotta N, et al. Active duodenal ulcer is associated with prolonged opening of the pylorus, increased retrograde transpyloric flow and delayed gastric emptying. Gastroenterology. 1998;114(4):A817–A817.
Avvari RK. Effect of local longitudinal shortening on the transport of luminal contents through small intestine. Acta Mechanica Sinica. 2019;35(1).
Avvari RK. Biomechanics of the small intestinal contractions, in management of digestive disorders, D.X. Qi, Editor. Intech Open. 2019.
Funding
(information that explains whether and by whom the research was supported). The author received no financial support for the research, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
(include appropriate disclosures). The author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avvari, R.K. Managing motility disorders of the gastrointestinal segment and obesity through electrical stimulation. Health Technol. 11, 1175–1189 (2021). https://doi.org/10.1007/s12553-021-00590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-021-00590-2