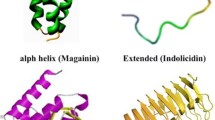
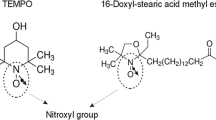
Abstract
The indiscriminate use of conventional antibiotics is leading to an increase in the number of resistant bacterial strains, motivating the search for new compounds to overcome this challenging problem. Antimicrobial peptides, acting only in the lipid phase of membranes without requiring specific membrane receptors as do conventional antibiotics, have shown great potential as possible substituents of these drugs. These peptides are in general rich in basic and hydrophobic residues forming an amphipathic structure when in contact with membranes. The outer leaflet of the prokaryotic cell membrane is rich in anionic lipids, while the surface of the eukaryotic cell is zwitterionic. Due to their positive net charge, many of these peptides are selective to the prokaryotic membrane. Notwithstanding this preference for anionic membranes, some of them can also act on neutral ones, hampering their therapeutic use. In addition to the electrostatic interaction driving peptide adsorption by the membrane, the ability of the peptide to perturb lipid packing is of paramount importance in their capacity to induce cell lysis, which is strongly dependent on electrostatic and hydrophobic interactions. In the present research, we revised the adsorption of antimicrobial peptides by model membranes as well as the perturbation that they induce in lipid packing. In particular, we focused on some peptides that have simultaneously acidic and basic residues. The net charges of these peptides are modulated by pH changes and the lipid composition of model membranes. We discuss the experimental approaches used to explore these aspects of lipid membranes using lipid vesicles and lipid monolayer as model membranes.



Similar content being viewed by others
References
Akashi K, Miyata H, Itoh H, Kinosita K (1996) Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys J 71:3242–3250
Alvares DS, Fanani ML, Ruggiero Neto J, Wilke N (2016) The interfacial properties of the peptide Polybia-MP1 and its interaction with DPPC are modulated by lateral electrostatic attractions. Biochim Biophys Acta Biomembr 1858:393–402
Alvares DS, Ruggiero Neto J, Ambroggio EE (2017) Phosphatidylserine lipids and membrane order precisely regulate the activity of Polybia-MP1 peptide. Biochim Biophys Acta Biomembr 1859:1067–1074
Ambroggio EE, Separovic F, Bowie J, Fidelio GD (2004) Surface behaviour and peptide-lipid interactions of the antibiotic peptides, Maculatin and Citropin. Biochim Biophys Acta Biomembr 1664:31–37
Ambroggio EE, Villarreal MA, Montich GG et al (2006) Interfacial properties of the M1 segment of the nicotinic acetylcholine receptor. Biophys Chem 121:171–176
Andreu D, Rivas L (1998) Animal antimicrobial peptides: an overview. Biopolymers 47:415–433
Angelova MI, Dimitrov DS (1986) Liposome electroformation. Faraday Discuss Chem Soc 81:303–311
Arias CA, Murray BE (2009) Antibiotic-resistant bugs in the 21st century — a clinical super-challenge. N Engl J Med 360:439–443
Arouri A, Kerth A, Dathe M, Blume A (2011) The binding of an amphipathic peptide to lipid monolayers at the air/water interface is modulated by the lipid headgroup structure. Langmuir 27:2811–2818
Bagheri A, Taheri-Araghi S, Ha BY (2015) How cell concentrations are implicated in cell selectivity of antimicrobial peptides. Langmuir 31:8052–8062
Bahar A, Ren D (2013) Antimicrobial peptides. Pharmaceuticals 6:1543–1575
Bechinger B (2015) The SMART model: soft membranes adapt and respond, also transiently, in the presence of antimicrobial peptides. J Pept Sci 21:346–355
Bechinger B, Lohner K (2006) Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta Biomembr 1758:1529–1539
Bechinger B, Zasloff M, Opella SJ (1992) Structure and interactions of magainin antibiotic peptides in lipid bilayers: a solid-state nuclear magnetic resonance investigation. Biophys J 62:12–14
Bernchou U, Ipsen JH, Simonsen AC (2009) Growth of solid domains in model membranes: quantitative image analysis reveals a strong correlation between domain shape and spatial position. J Phys Chem B 113:7170–7177
Birdi KS (2006) Self-assembly monolayer structures of lipids and macromolecules at interfaces. Springer, New York
Boisselier É, Demers É, Cantin L, Salesse C (2017) How to gather useful and valuable information from protein binding measurements using Langmuir lipid monolayers. Adv Colloid Interf Sci 243:60–76
Bouffioux O, Berquand A, Eeman M et al (2007) Molecular organization of surfactin-phospholipid monolayers: effect of phospholipid chain length and polar head. Biochim Biophys Acta Biomembr 1768:1758–1768
Brockman H (1999) Lipid monolayers: why use half a membrane to characterize protein-membrane interactions? Curr Opin Struct Biol 9:438–443
Chen Y, Vasil AI, Rehaume L et al (2006) Comparison of biophysical and biologic properties of alpha-helical enantiomeric antimicrobial peptides. Chem Biol Drug Des 67:162–173
Clausell A, Busquets MA, Pujol M et al (2004) Polymyxin B-lipid interactions in Langmuir-Blodgett monolayers of Escherichia Coli lipids: a thermodynamic and atomic force microscopy study. Biopolymers 75:480–490
da Silva AVR, De Souza BM, Dos Santos Cabrera MP et al (2014) The effects of the C-terminal amidation of mastoparans on their biological actions and interactions with membrane-mimetic systems. Biochim Biophys Acta 1838:2357–2368
Dathe M, Wieprecht T (1999) Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta Biomembr 1462:71–87
Dennison SR, Harris F, Phoenix DA (2007) The interactions of aurein 1.2 with cancer cell membranes. Biophys Chem 127:78–83
Dennison SR, Morton LHG, Harris F, Phoenix DA (2008) The impact of membrane lipid composition on antimicrobial function of an alpha-helical peptide. Chem Phys Lipids 151:92–102
Dennison SR, Morton LHG, Shorrocks AJ et al (2009) A study on the interactions of Aurein 2.5 with bacterial membranes. Colloids Surf B 68:225–230
Dennison SR, Harris F, Phoenix DA (2010) A Langmuir approach using on monolayer interactions to investigate surface active peptides. Protein Pept Lett 17:1363–1375
Dennison SR, Harris F, Phoenix DA (2014) Langmuir–Blodgett approach to investigate antimicrobial peptide–membrane interactions. Adv Planar Lipid Bilayers Liposomes 20:83–110
Devaux PF (1991) Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30:1163–1173
Devaux PF (1992) Protein involvement in transmembrane lipid asymmetry. Annu Rev Biophys Biomol Struct 21:417–439
Dimova R, Aranda S, Bezlyepkina N et al (2006) A practical guide to giant vesicles. Probing the membrane nanoregime via optical microscopy. J Phys Condens Matter 18:S1151–S1176
Dos Santos Cabrera MP, De Souza BM, Fontana R et al (2004) Conformation and lytic activity of eumenine mastoparan: a new antimicrobial peptide from wasp venom. J Pept Res 64:95–103
Dos Santos Cabrera MP, Alvares DS, Leite NB et al (2011) New insight into the mechanism of action of wasp mastoparan peptides: Lytic activity and clustering observed with giant vesicles. Langmuir 27:10805–10813
Dos Santos Cabrera MP, Arcisio-Miranda M, Gorjão R et al (2012) Influence of the bilayer composition on the binding and membrane disrupting effect of polybia-MP1, an antimicrobial mastoparan peptide with leukemic T-lymphocyte cell selectivity. Biochemistry 51:4898–4908
Eeman M, Deleu M (2010) From biological membranes to biomimetic model membranes. Biotechnol Agron Soc Environ 14:719–736
Eeman M, Berquand A, Dufrêne YF et al (2006) Penetration of surfactin into phospholipid monolayers: Nanoscale interfacial organization. Langmuir 22:11337–11345
Epand RM, Vogel HJ (1999) Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta Biomembr 1462:11–28
Epand RF, Wang G, Berno B, Epand RM (2009) Lipid segregation explains selective toxicity of a series of fragments derived from the human cathelicidin LL-37. Antimicrob Agents Chemother 53:3705–3714
Epand RF, Maloy WL, Ramamoorthy A, Epand RM (2010) Probing the “charge cluster mechanism” in amphipathic helical cationic antimicrobial peptides. Biochemistry 49:4076–4084
Fadok VA, Voelker DR, Campbell PA et al (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:2207–2216
Fadok VA, Bratton DL, Frasch SC et al (1998) The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ 5:551–562
Fidelio GD, Maggio B, Cumar FA (1986) Interaction of melittin with glycosphingolipids and phospholipids in mixed monolayers at different temperatures. Effect of the lipid physical state. Biochim Biophys Acta Biomembr 862:49–56
Fischer A, Lösche M, Möhwald H, Sackmann E (1984) On the nature of the lipid monolayer phase transition. J Phys Lett 45:785–791
Fjell CD, Hiss JA, Hancock REW, Schneider G (2012) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51
Fošnarič M, Iglič A, May S (2006) Influence of rigid inclusions on the bending elasticity of a lipid membrane. Phys Rev E 74:1–12
Grimsley GR, Scholtz JM, Pace CN (2009) A summary of the measured pK values of the ionizable groups in folded proteins. Protein Sci 18:247–251
Hädicke A, Blume A (2016) Binding of the cationic peptide (KL)4K to lipid Monolayers at the air–water Interface: effect of lipid Headgroup charge, Acyl chain length, and Acyl chain saturation. J Phys Chem B 120:3880–3887
Hancock RE, Falla T, Brown M (1995) Cationic bactericidal peptides. Adv Microb Physiol 37:135–175
Haney EF, Nathoo S, Vogel HJ, Prenner EJ (2010) Induction of non-lamellar lipid phases by antimicrobial peptides: a potential link to mode of action. Chem Phys Lipids 163:82–93
He K, Ludtke SJ, Huang HW, Worcester DL (1995) Antimicrobial peptide pores in membranes detected by neutron in-plane scattering. Biochemistry 34:15614–15618
Heckl WM, Losche M, Cadenhead DA, Mohwald H (1986) Electrostatically induced growth of spiral lipid domains in the presence of cholesterol. Eur Biophys J Biophys Lett 14:11–17
Henon S, Meunier J (1991) Microscope at the Brewster angle: direct observation of first-order phase transitions in monolayer. Rev Sci Instrum 62:936–939
Hoenig D, Moebius D (1991) Direct visualization of monolayers at the air-water interface by Brewster angle microscopy. J Phys Chem 95:4590–4592
Hope MJ, Bally MB, Webb G, Cullis PR (1985) Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim Biophys Acta Biomembr 812:55–65
Hope MJ, Bally MB, Mayer LD et al (1986) Generation of multilamellar and unilamellar phospholipid vesicles. Chem Phys Lipids 40:89–107
Huang HW, Chen FY, Lee MT (2004) Molecular mechanism of peptide-induced pores in membranes. Phys Rev Lett 92:198304–198301
Ishii H, Mori T, Shiratsuchi A et al (2005) Distinct localization of lipid rafts and externalized phosphatidylserine at the surface of apoptotic cells. Biochem Biophys Res Commun 327:94–99
Islam MZ, Alam JM, Tamba Y et al (2014) The single GUV method for revealing the functions of antimicrobial, pore-forming toxin, and cell-penetrating peptides or proteins. Phys Chem Chem Phys 16:15752–15767
Jin Y, Hammer J, Pate M et al (2005) Antimicrobial activities and structures of two linear cationic peptide families with various amphipathic β-sheet and α-helical potentials. Antimicrob Agents Chemother 49:4957–4964
Jing W, Hunter HN, Hagel J, Vogel HJ (2003) The structure of the antimicrobial peptide ac-RRWWRF-NH2 bound to micelles and its interactions with phospholipid bilayers. J Pept Res 61:219–229
Kaganer V, Möhwald H, Dutta P (1999) Structure and phase transitions in Langmuir monolayers. Rev Mod Phys 71:779–819
Klocek G, Schulthess T, Shai Y, Seelig J (2009) Thermodynamics of melittin binding to lipid bilayers. Aggregation and pore formation. Biochemistry 48:2586–2596
Ladokhin AS, Wimley WC, White SH (1995) Leakage of membrane vesicle contents: determination of mechanism using fluorescence requenching. Biophys J 69:1964–1971
Leite NB, Da Costa LC, Alvares DS et al (2011) The effect of acidic residues and amphipathicity on the lytic activities of mastoparan peptides studied by fluorescence and CD spectroscopy. Amino Acids 40:91–100
Leite NB, Aufderhorst-Roberts A, Palma MS et al (2015) PE and PS lipids synergistically enhance membrane Poration by a peptide with anticancer properties. Biophys J 109:936–947
Lheveder C, Hénon S, Meunier J (2000) Brewster angle microscopy. In: Baszkin A, Norde W (eds) Physical chemistry of biological interfaces. Marcel Dekker, New York, p 848
Lösche M, Sackmann E, Möhwald H (1983) A fluorescence microscopic study concerning the phase diagram of phospholipids. Ber Bunsenges Phys Chem 87:848–852
Maget-Dana R (1999) The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim Biophys Acta Biomembr 1462:109–140
Maget-Dana R, Lelièvre D, Brack A (1999) Surface active properties of amphiphilic sequential isopeptides: comparison between α-helical and β-sheet conformations. Biopolymers 49:415–423
Malanovic N, Lohner K (2015) Gram-positive bacterial cell envelopes: the impact on the activity of antimicrobial peptides. Biochim Biophys Acta 1858:936–946
Maltseva E, Shapovalov VL, Möhwald H, Brezesinski G (2006) Ionization state and structure of L-1,2-dipalmitoylphosphatidylglycerol monolayers at the liquid/air interface. J Phys Chem B 110:919–926
Mangiarotti A, Wilke N (2015) Energetics of the phase transition in free-standing versus supported lipid membranes. J Phys Chem B 119:8718–8724
Marassi FM, Opella SJ (2000) A solid-state NMR index of helical membrane protein structure and topology. J Magn Reson 144:150–155
Marsh D (1996) Lateral pressure in membranes. Biochim Biophys Acta Rev Biomembr 1286:183–223
Marshall SH, Arenas G (2003) Antimicrobial peptides: a natural alternative to chemical antibiotics and a potential for applied biotechnology. Electron J Biotechnol 6:271–284
Matsuzaki K, Murase O, Fujii N, Miyajima K (1996a) An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 35:11361–11368
Matsuzaki K, Yoneyama S, Murase O, Miyajima K (1996b) Transbilayer transport of ions and lipids coupled with mastoparan X translocation. Biochemistry 35:8450–8456
May S, Harries D, Ben-shaul A (2000) Lipid Demixing and protein-protein interactions in the adsorption of charged proteins on mixed membranes. Biophys J 79:1747–1760
McConlogue CW, Vanderlick TK (1997) A close look at domain formation in DPPC Monolayers. Langmuir 13:7158–7164
McElhaney RN (1982) The use of differential scanning calorimetry and differential thermal analysis in studies of model and biological membranes. Chem Phys Lipids 30:229–259
McElhaney RN (1986) Differential scanning calorimetric studies of lipid-protein interactions in model membrane systems. Biochim Biophys Acta 864:361–421
Miller A, Mohwald H (1987) Diffusion limited growth of crystalline domains in phospholipid monolayers. J Chem Phys 86:4258–4265
Mohwald H (1990) Phospholipid and Phospholipid-protein Monolayers at the air/water Interface. Annu Rev Phys Chem 41:441–476
Möhwald H (1995) Phospholipid monolayers. In: Lipowsky R, Sackmann E (eds) Handbook of biological physics, vol 1. Elsevier, Netherlands, pp 161–211
Mottola M, Wilke N, Benedini L et al (2013) Ascorbyl palmitate interaction with phospholipid monolayers: electrostatic and rheological preponderancy. Biochim Biophys Acta Biomembr 1828:2496–2505
Mularski A, Wilksch JJ, Wang H et al (2015) Atomic force microscopy reveals the Mechanobiology of Lytic peptide action on bacteria. Langmuir 31:6164–6171
Mura M, Dennison SR, Zvelindovsky AV, Phoenix DA (2013) Aurein 2.3 functionality is supported by oblique orientated alpha-helical formation. Biochim Biophys Acta Biomembr 1828:586–594
Neville F, Cahuzac M, Konovalov O et al (2006) Lipid headgroup discrimination by antimicrobial peptide LL-37: insight into mechanism of action. Biophys J 90:1275–1287
Nguyen LT, Haney EF, Vogel HJ (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 29:464–472
O’Neill J (2015) Tackling a global health crisis: initial steps The Review on Antimicrobial Resistance Chaired The Review on Antimicrobial Resistance, Chaired by Jim O’Neill
Parente RA, Nir S, Szoka FC (1990) Mechanism of leakage of phospholipid vesicle contents induced by the peptide GALA. Biochemistry 29:8720–8728
Patel H, Tscheka C, Heerklotz H (2009) Characterizing vesicle leakage by fluorescence lifetime measurements. Soft Matter 5:2849
Pokorny A, Birkbeck TH, Almeida PFF (2002) Mechanism and kinetics of δ-lysin interaction with phospholipid vesicles. Biochemistry 41:11044–11056
Puff N, Angelova MI (2006) Lipid vesicles-development and applications for studding membrane heterogeneity and interactions. Adv Planar Lipid Bilayer Liposomes 5:173–228
Quinn PJ (2010) A lipid matrix model of membrane raft structure. Prog Lipid Res 49:390–406
Rautenbach M, Gerstner GD, Vlok NM et al (2006) Analyses of dose–response curves to compare the antimicrobial activity of model cationic α-helical peptides highlights the necessity for a minimum of two activity parameters. Anal Biochem 350:81–90
Riske KA (2015) Optical microscopy of giant vesicles as a tool to reveal the mechanism of action of antimicrobial peptides and the specific case of Gomesin. Adv Planar Lipid Bilayer Liposomes 21:99–129
Rosetti CM, Mangiarotti A, Wilke N (2017) Sizes of lipid domains: what do we know from artificial lipid membranes? What are the possible shared features with membrane rafts in cells? Biochim Biophys Acta Biomembr 1859:789–802
Rothman JE, Lenard J (1977) Membrane asymmetry. Science 195:743–753
Seelig A (1990) Substance P and antagonists. Surface activity and molecular shapes. Biochim Biophys Acta Biomembr 1030:111–118
Sengupta D, Leontiadou H, Mark AE, Marrink SJ (2008) Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta Biomembr 1778:2308–2317
Sezgin E, Levental I, Mayor S, Eggeling C (2017) The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol 18:361–374
Sforça ML, Oyama S, Canduri F et al (2004) How C-terminal carboxyamidation alters the biological activity of peptides from the venom of the eumenine solitary wasp. Biochemistry 43:5608–5617
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta Biomembr 1462:55–70
Silvestro L, Axelsen PH (2000) Membrane-induced folding of Cecropin A. Biophys J 79:1465–1477
Spaar A, Münster C, Salditt T (2004) Conformation of peptides in lipid membranes studied by x-ray grazing incidence scattering. Biophys J 87:396–407
Stafford JH, Thorpe PE (2011) Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia 13:299–308
Stefaniu C, Brezesinski G, Möhwald H (2014) Langmuir monolayers as models to study processes at membrane surfaces. Adv Colloid Interf Sci 208:197–213
Tamba Y, Terashima H, Yamazaki M (2011) A membrane filtering method for the purification of giant unilamellar vesicles. Chem Phys Lipids 164:351–358
Travkova OG, Brezesinski G (2013) Adsorption of the antimicrobial peptide arenicin and its linear derivative to model membranes - a maximum insertion pressure study. Chem Phys Lipids 167–168:43–50
Utsugi T, Schroit AJ, Connor J et al (1991) Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res 51:3062–3066
Vanderlick TK, Möhwald H (1990) Mode selection and shape transition of phospholipid monolayer domains. J Phys Chem 94:886–890
Vega Mercado F, Maggio B, Wilke N (2011) Phase diagram of mixed monolayers of stearic acid and dimyristoylphosphatidylcholine. Effect of the acid ionization. Chem Phys Lipids 164:386–392
Vega Mercado F, Maggio B, Wilke N (2012) Modulation of the domain topography of biphasic monolayers of stearic acid and dimyristoyl phosphatidylcholine. Chem Phys Lipids 165:232–237
Wade D, Boman A, Wåhlin B et al (1990) All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A 87:4761–4765
Wang G (2014) Human antimicrobial peptides and proteins. Pharmaceuticals (Basel) 7:545–594
Wang K, Zhang B, Zhang W et al (2008) Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides 29:963–968
Wang K, Yan J, Zhang B et al (2009) Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett 278:65–72
Wang K, Yan J, Chen R et al (2012) Membrane-active action mode of polybia-CP, a novel antimicrobial peptide isolated from the venom of Polybia paulista. Antimicrob Agents Chemother 56:3318–3323
Weinberger A, Tsai F-C, Koenderink GH et al (2013) Gel-assisted formation of Giant Unilamellar vesicles. Biophys J 105:154–164
Weis RM, McConnell HM (1984) Two-dimensional chiral crystals of phospholipid. Nature 310:47–49
Wilke N (2014) Lipid monolayers at the air-water interface: a tool for understanding electrostatic interactions and rheology in biomembranes. Adv Planar Lipid Bilayer Liposomes 20:51–81
Wimley WC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5:905–917
Wimley WC, Hristova K (2011) Antimicrobial peptides: successes, challenges and unanswered questions. J Membr Biol 239:27–34
Worthman LA, Nag K, Davis PJ, Keough KM (1997) Cholesterol in condensed and fluid phosphatidylcholine monolayers studied by epifluorescence microscopy. Biophys J 72:2569–2580
Yamazaki M, Tamba Y (2005) The single GUV method for probing biomembrane structure and function. e-J Surf Sci Nanotechnol 3:218–227
Yandek LE, Pokorny A, Almeida PFF (2009) Wasp mastoparans follow the same mechanism as the cell-penetrating peptide transportan 10. Biochemistry 48:7342–7351
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Yeung ATY, Gellatly SL, Hancock REW (2011) Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci 68:2161–2176
Zanin LMP, Alvares DS, Juliano MA et al (2013) Interaction of a synthetic antimicrobial peptide with model membrane by fluorescence spectroscopy. Eur Biophys J 42:819–831
Zanin LPM, de Araujo AS, Juliano MA et al (2016) Effects of N-terminus modifications on the conformation and permeation activities of the synthetic peptide L1A. Amino Acids 48:1433–1444
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zemel A, Ben-Shaul A, May S (2008) Modulation of the spontaneous curvature and bending rigidity of lipid membranes by interfacially adsorbed amphipathic peptides. J Phys Chem B 112:6988–6996
Acknowledgments
The authors acknowledge financial support from São Paulo Research Foundation - FAPESP (J.R.N. Grants #2015/25619-9 and D.S.A has a post-doctorate fellowship, grants #2015/25620-7). J.R.N. is a researcher for Brazil’s National Council for Scientific and Technological Development (CNPq). T.G.V. has a PhD fellowship from CNPq. DSA thanks UNESP and CAPES for former scholarships. The authors thank Dr. Paul Andrew Beales from the University of Leeds (UK) and Dr. Ernesto E. Ambroggio from the University of Cordoba (Argentina) with whom the FCM experiments were performed in collaborative projects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dayane S. Alvares declares that she has no conflicts of interest. Taisa Giordano Viegas declares that she has no conflicts of interest. João Ruggiero Neto declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This article is part of a Special Issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri
Rights and permissions
About this article
Cite this article
Alvares, D.S., Viegas, T.G. & Ruggiero Neto, J. Lipid-packing perturbation of model membranes by pH-responsive antimicrobial peptides. Biophys Rev 9, 669–682 (2017). https://doi.org/10.1007/s12551-017-0296-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-017-0296-0