Abstract
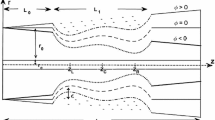
A novel eccentric stenotic channel was manufactured to provide an in vivo physical environment simulating an atherosclerotic artery. It was composed of an elastic wall containing a soft semi-cylindrical volume that simulated a lipid core. Wall strain and fluid shear stress distributions were computed using a finite element method incorporating fluid structure interaction. The particle image velocity measurement method was extended to measure wall deformation and wall deformation and flow velocity were simultaneously measured to validate the computational method. The maximum deformation was found to occur in the distal shoulder, while the maximum flow wall shear stress was skewed to the proximal shoulder of the lipid core. Measured maximum wall deformations and flow velocities agreed with the computational results within a 10% margin of error. The wall shear stress on the stenotic wall varied from 0 to 1.2 Pa. The maximum shear strain in the longitudinal plane and the normal strain in the transverse plane for the Core 1 model (Young’s modulus 50 kPa) were 3.8% and 0.7%, respectively. The maximum shear and normal wall strains for the Core 2 model (Young’s modulus 200 kPa) were 34% and 14% lower. These wall stress and strain values corresponded with those found in human atherosclerotic arteries. The developed channel could provide spatial and temporal variations as well as magnitudes of wall shear stress and strain observed in atherosclerotic arteries.












Similar content being viewed by others
References
Davies, P. F. (1995). Flow-mediated endothelial mechanotransduction. Physiological Reviews, 75(3), 519–560.
Dhawan, S. S., Avati Nanjundappa, R. P., Branch, J. R., Taylor, W. R., Quyyumi, A. A., Jo, H., et al. (2010). Shear stress and plaque development. Expert Review of Cardiovascular Therapy, 8(4), 545–556.
Koskinas, K. C., Chatzizisis, Y. S., Baker, A. B., Edelman, E. R., Stone, P. H., & Feldman, C. L. (2009). The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Current Opinion in Cardiology, 24(6), 580–590.
Giddens, D. P., Zarins, C. K., & Glagov, S. (1993). The role of fluid mechanics in the localization and detection of atherosclerosis. Journal of Biomechanical Engineering, 115(4B), 588–594.
Glagov, S., Bassiouny, H. S., Giddens, D. P., & Zarins, C. K. (1995). Pathobiology of plaque modeling and complication. Surgical Clinics of North America, 75(4), 545–556.
Dai, G., Kaazempur-Mofrad, M. R., Natarajan, S., Zhang, Y., Vaughn, S., Blackman, B. R., et al. (2004). Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and-resistant regions of human vasculature. Proceedings of the National Academy of Sciences, 101(41), 14871–14876.
Slager, C. J., Wentzel, J. J., Gijsen, F. J. H., Schuurbiers, J. C. H., Van der Wal, A. C., Van der Steen, A. F. W., et al. (2005). The role of shear stress in the generation of rupture-prone vulnerable plaques. Nature Reviews Cardiology, 2(8), 401–407.
Mattsson, E. J., Kohler, T. R., Vergel, S. M., & Clowes, A. W. (1997). Increased blood flow induces regression of intimal hyperplasia. Arteriosclerosis, Thrombosis, and Vascular Biology, 17(10), 2245–2249.
Birukov, K. G., Bardy, N., Lehoux, S., Merval, R., Shirinsky, V. P., & Tedgui, A. (1998). Intraluminal pressure is essential for the maintenance of smooth muscle caldesmon and filamin content in aortic organ culture. Arteriosclerosis, Thrombosis, and Vascular Biology, 18(6), 922–927.
Chapman, G. B., Durante, W., Hellums, J. D., & Schafer, A. I. (2000). Physiological cyclic stretch causes cell cycle arrest in cultured vascular smooth muscle cells. American Journal of Physiology-Heart and Circulatory Physiology, 278(3), H748–H754.
Wallace, C. S., Champion, J. C., & Truskey, G. A. (2007). Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Annals of Biomedical Engineering, 35(3), 375–386.
McWhorter, F. Y., Davis, C. T., & Liu, W. F. (2015). Physical and mechanical regulation of macrophage phenotype and function. Cellular and Molecular Life Sciences, 72(7), 1303–1316.
Ballotta, V., Driessen-Mol, A., Bouten, C. V., & Baaijens, F. P. (2014). Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials, 35(18), 4919–4928.
Kim, K., Han, D. Y., Chang, K., & Lee, W. G. (2018). Design and manufacturing of a uniform, massively parallel fluid distributor for automated cell culture bioreactor. International Journal of Precision Engineering and Manufacturing, 19(3), 417–424.
Hwang, J., Cho, Y. H., Park, M. S., & Kim, B. H. (2019). Microchannel fabrication on glass materials for microfluidic devices. International Journal of Precision Engineering and Manufacturing, 20(3), 479–495.
Ruel, J., Lemay, J., Dumas, G., Doillon, C., & Charara, J. (1995). Development of a parallel plate flow chamber for studying cell behavior under pulsatile flow. ASAIO Journal, 41(4), 876–883.
Bacabac, R. G., Smit, T. H., Cowin, S. C., Van Loon, J. J., Nieuwstadt, F. T., Heethaar, R., et al. (2005). Dynamic shear stress in parallel-plate flow chambers. Journal of Biomechanics, 38(1), 159–167.
Chappell, D. C., Varner, S. E., Nerem, R. M., Medford, R. M., & Alexander, R. W. (1998). Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circulation Research, 82(5), 532–539.
Brown, T. D. (2000). Techniques for mechanical stimulation of cells in vitro: a review. Journal of Biomechanics, 33(1), 3–14.
Chau, L., Doran, M., & Cooper-White, J. (2009). A novel multishear microdevice for studying cell mechanics. Lab on a Chip, 9(13), 1897–1902.
Heo, K. S., Fujiwara, K., & Abe, J. I. (2014). Shear stress and atherosclerosis. Molecules and Cells, 37(6), 435–440.
Redmond, E. M., Cahill, P. A., & Sitzmann, J. V. (1995). Perfused transcapillary smooth muscle and endothelial cell co-culture—A novelin vitro model. Vitro Cellular & Developmental Biology-Animal, 31(8), 601–609.
Matsumoto, T., Yung, Y. C., Fischbach, C., Kong, H. J., Nakaoka, R., & Mooney, D. J. (2007). Mechanical strain regulates endothelial cell patterning in vitro. Tissue Engineering, 13(1), 207–217.
Gerstmair, A., Fois, G., Innerbichler, S., Dietl, P., & Felder, E. (2009). A device for simultaneous live cell imaging during uni-axial mechanical strain or compression. Journal of Applied Physiology, 107, 613–620.
Lee, J., Wong, M., Smith, Q., & Baker, A. B. (2013). A novel system for studying mechanical strain waveform-dependent responses in vascular smooth muscle cells. Lab on a Chip, 13(23), 4573–4582.
Berardi, D. E., & Tarbell, J. M. (2009). Stretch and shear interactions affect intercellular junction protein expression and turnover in endothelial cells. Cellular and Molecular Bioengineering, 2(3), 320–331.
Punchard, M. A., O’Cearbhaill, E. D., Mackle, J. N., McHugh, P. E., Smith, T. J., Stenson-Cox, C., et al. (2009). Evaluation of human endothelial cells post stent deployment in a cardiovascular simulator in vitro. Annals of Biomedical Engineering, 37(7), 1322–1330.
Estrada, R., Giridharan, G. A., Nguyen, M. D., Prabhu, S. D., & Sethu, P. (2011). Microfluidic endothelial cell culture model to replicate disturbed flow conditions seen in atherosclerosis susceptible regions. Biomicrofluidics, 5(3), 032006.
Keane, R. D., & Adrian, R. J. (1992). Theory of cross-correlation analysis of PIV images. Applied Scientific Research, 49(3), 191–215.
Sadek, S., Iskander, M. G., & Liu, J. (2003). Accuracy of digital image correlation for measuring deformations in transparent media. Journal of Computing in Civil Engineering, 17(2), 88–96.
Sabass, B., Gardel, M. L., Waterman, C. M., & Schwarz, U. S. (2008). High resolution traction force microscopy based on experimental and computational advances. Biophysical Journal, 94(1), 207–220.
Ng, S. S., Li, C., & Chan, V. (2011). Experimental and numerical determination of cellular traction force on polymeric hydrogels. Interface focus, 1(5), 777–791.
Kang, M. J., & Rhee, K. (2017). Computation of stress field in a polymer scaffold from optically measured deformation field using particle images. International Journal of Precision Engineering and Manufacturing, 18(7), 1021–1026.
Charonko, J., Karri, S., Schmieg, J., Prabhu, S., & Vlachos, P. (2009). In vitro, time-resolved PIV comparison of the effect of stent design on wall shear stress. Annals of Biomedical Engineering, 37(7), 1310–1321.
Wang, Z., Volinsky, A. A., & Gallant, N. D. (2014). Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. Journal of Applied Polymer Science, 131(22), 41050.
Gijsen, F., van der Giessen, A., van der Steen, A., & Wentzel, J. (2013). Shear stress and advanced atherosclerosis in human coronary arteries. Journal of Biomechanics, 46(2), 240–247.
Li, Y. S. J., Haga, J. H., & Chien, S. (2005). Molecular basis of the effects of shear stress on vascular endothelial cells. Journal of Biomechanics, 38(10), 1949–1971.
Polacheck, W. J., Li, R., Uzel, S. G., & Kamm, R. D. (2013). Microfluidic platforms for mechanobiology. Lab on a Chip, 13(12), 2252–2267.
Maurice, R. L., Fromageau, J., Brusseau, É., Finet, G., Rioufol, G., & Cloutier, G. (2007). On the potential of the lagrangian estimator for endovascular ultrasound elastography: In vivo human coronary artery study. Ultrasound in Medicine and Biology, 33(8), 1199–1205.
Lee, R. T., Schoen, F. J., Loree, H. M., Lark, M. W., & Libby, P. (1996). Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis: implications for plaque rupture. Arteriosclerosis, Thrombosis, and Vascular Biology, 16(8), 1070–1073.
Chung, S., Sudo, R., Mack, P. J., Wan, C. R., Vickerman, V., & Kamm, R. D. (2009). Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab on a Chip, 9(2), 269–275.
Zheng, Y., Chen, J., Craven, M., Choi, N. W., Totorica, S., Diaz-Santana, A., et al. (2012). In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the National Academy of Sciences, 109(24), 9342–9347.
Acknowledgements
This work was supported by the Research Fund (NRF-2017R1A2B4004439).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, M.H., Chhai, P. & Rhee, K. Analysis of Flow and Wall Deformation in a Stenotic Flexible Channel Containing a Soft Core, Simulating Atherosclerotic Arteries. Int. J. Precis. Eng. Manuf. 20, 1047–1056 (2019). https://doi.org/10.1007/s12541-019-00122-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12541-019-00122-z