Abstract
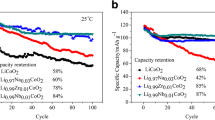
In an attempt to understand the effect of synthesis temperature upon surface morphology and lithium diffusion kinetics of LiCoO2, the compound was synthesized at four different temperatures, viz., 600, 700, 800 and 900 °C using a novel gelatin-assisted combustion method. LiCoO2 synthesized at 800 °C is found to be a mixture of rhombohedral and cubic LiCoO2 and a temperature of 900 °C leads to the formation of cubic LiCo2O4 compound, thus favoring lower temperatures such as 600 and 700 °C to prepare phase pure rhombohedral LiCoO2. Cyclic voltametry and impedance spectral studies evidence that LiCoO2 synthesized at 600 °C exhibits better electrochemical cycling behavior and considerably reduced internal resistance upon cycling, which are substantiated further from the higher lithium diffusion coefficient value. The study demonstrates the possibility and superiority of synthesizing electrochemically active LiCoO2 with preferred surface morphology and better lithium diffusion kinetics at a relatively lower temperature of 600 °C, using a gelatin-assisted combustion method.
Similar content being viewed by others
References
D. W. Murphy, F. J. DiSalvo, J. N. Carides, and J. V. Waszczak, Mater. Res. Bull. 13, 1395 (1978).
J. M. Tarascon, W. R. Mckinnon, F. Coowar, T. N. Bowmer, G. Amatucci, and D. Guyomard, J. Electrochem. Soc. 141, 1421 (1994).
J. M. Tarascon, E. Wang, F. K. Shokoohi, W. R. McKinnon, and S. Colson, J. Electrochem. Soc. 138, 2859 (1991).
V. G. Kumar, J. S. Gnanaraj, S. Ben-David, D. M. Pickup, E. R. H. Van-Eck, A. Gedanken, and D. Aurbach, Chem. Mater. 15, 4211 (2003).
L. H. Yu, H. X. Yang, X. P. Ai, and Y. L. Cao, J. Phys. Chem. B 109, 1148 (2005).
A. S. Aricq, P. Bruce, B. Scrosati, J. M. Tarascon, and W. Van Schalkwijc, Nature Mater. 4, 366 (2005).
O. A. Shlyakhtin, Y. S. Yoon, and Y. J. Oh, J. Eur. Ceram. Soc. 23, 1893 (2003).
S. P. S. Prabaharan, M. S. Michael, T. P. Kumar, A. Mani, K. Athinarayanaswamy, and R. Gangadharan, J. Mater Chem. 5, 1035 (1995).
M. Yoshino, Y. Xia, K. Ikeda, and H. Noguchi, Mat. Res. Soc. Symp. Proc. 393, 91 (1995).
Z. Jiang and K. M. Abraham, J. Electrochem. Soc. 143, 1591 (1996).
T. Nohma, H. Kurokawa, M. Uehara, M. Takahasi, K. Nishito, and T. Saito, J. Power Sources 54, 522 (1995).
S. Valanarasu, R. Chandramohan, J. Thirumalai, and T. A. Vijayan, J. Sci. Res. 2, 443 (2010).
S. Mohd Ali, M. Azrulnizam, and S. S. Khairul, J. Solid St. Sci. and Technol. Letters 12, 77 (2005).
E. I. Santiago, V. C. Andrade, C. O. Paiva-Santos, and L. V. O. Bulhoes, Solid State Ionics 158, 91 (2003).
M. Jo, H. S. Hong, J. Choo, and J. Cho, J. Electrochem. Soc. 156, A430 (2009).
P. Kalyani, N. Kalaiselvi, and N. Muniyandi, J. Power Sources 111, 232 (2002).
C. Julien, M. A. Camacho-Lopez, T. Mohan, S. Chitra, P. Kalyani, and S. Gopukumar, Solid State Ionics 135, 241 (2000).
T. Ohzuku, A. Ueda, M. Nagayama, Y. Iwakoshi, and H. Komori, Electrochim. Acta. 38, 1159 (1993).
H. Sato, H. D. Takahashi, T. Nishina, and I. Uchida, J. Power Sources 68, 540 (1997).
A. J. Bard and L. R. Faulkner, Electrochemical Methods, Wiley, New York (1980).
D. W. Shin, J. W. Choi, W. K. Choi, Y. S. Cho, and S. J. Yoon, Electrochem. Commun. 11, 695 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakshmanan, R., Gangulibabu, Bhuvaneswari, D. et al. Temperature dependent surface morphology and lithium diffusion kinetics of LiCoO2 cathode. Met. Mater. Int. 18, 249–255 (2012). https://doi.org/10.1007/s12540-012-2008-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-012-2008-4