Abstract
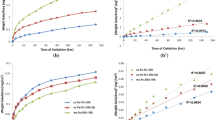
In this study, cast bulk specimens and powder sintered specimens were produced from a Fe-22% Cr-5.8% Al alloy, which has been drawing industrial attention for its outstanding high-thermal and oxidation resistance as well as for its applicability as a filter and support material. The high-temperature oxidation behaviors of the specimens were compared and analyzed. Two types of specimens were subjected to 24-hour isothermal oxidation (temperatures of 900 °C, 1000 °C, and 1100 °C and a gas atmosphere of 79% N2+21% O2) to understand their high-temperature oxidation properties. The oxidized specimens were examined via X-ray, SEM, FE-SEM, and EPMA for microstructure and oxide analyses. The results of the oxidation tests showed that both bulk and powder-sintered specimens gained weight as temperature increased, with the powder sintered specimens gaining far more weight than the bulk specimens. As the temperature increased, the bulk specimens showed sequential formation of Al 2 O3, Cr2O3, and Fe3O4 oxide layers. The stable Al2O3 on the surface of the bulk specimens was found to play a role in arresting the rapid growth of oxide layers. In the powder-sintered specimens, however, the internal diffusion of O2 easily took place along the powder interfaces. As a result, far more oxidation occurred in the powder-sintered specimens than in the bulk specimens. Cracks and disintegration of the oxide layers were also found to have occurred as the temperature increased. The high-temperature oxidation mechanisms of bulk and powder-sintered materials in relation to temperature increase are further discussed.
Similar content being viewed by others
References
W. Wegener and G. Borchardt, Oxidation of Metals 36, 339 (1991).
H. W. Pickering, J. Electrochem, Soc. 119, 641 (1972).
T. W. Kim, S. H. Jo, I. Y. Ko, J. M. Doh, J. K. Yoon, I. J. Shon, Kor. J. Met. Mater. 48, 981 (2010).
P. D. Hodgson and R. Jackson, Met. Forum 4, 192 (1981).
R. Cueff, H. Buscail, E. Caudron, C. Issartel, and F. Riffard, Corros, Sci. 45, 1815 (2003).
B. G. Moon, S. C. Kang, and G. M. Kim, J. Corros Sci. Soc. of Korea 25, 149 (1996).
F. Clemendot, J. M. Gras, and J.C. van Duysen, G. Zacharie, Corrs. Sci. 35, 5 (1993).
S. C. Choi, S. H. Kim, D. B. Lee, and H. S. Park, J. Korean Inst. Met. & Mater. 35, 145 (1997).
K. S. Lee, Y. C. Shin, K. H. Oh, W. W Park, and H. Y. Na, J. Korean Inst. Met. & Mater. 34, 943 (1996).
S. Hiroshi, K. Masski, and Y. Keiichi, Kawasaki Steel Technical Report. 31, 1 (1994).
Y. Zhou, X. Q. Zuo, J. H. Sun, J. Mei, and J. L. Sun, Mater. Sci. Eng. A, 457, 329 (2007).
W. J. Quadakkers, J. Jedlinski, K. Schmidt, M. Krasovec, G. Borchardt, and H. Nikel, Applied Surface Science 47, 261 (1991).
D. R. Sigler, Oxidation of Metals 36, 57 (1991).
C. Wagner, Z. Phys. Chem. 21, 25 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SY., Choi, SH., Yun, JY. et al. High-temperature oxidation behaviors of Fe-Cr-Al bulk and powder-sintered materials. Met. Mater. Int. 17, 983–992 (2011). https://doi.org/10.1007/s12540-011-6017-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-011-6017-5