Abstract
Due to the increasing challenge of meeting human demands for metals from land-based resources, interest in extracting mineral ores from the deep sea has gained momentum in recent years. Targeted mining of deep-seabed minerals could have adverse effects on the associated ecosystems, but knowledge on the biological communities found there, their structure and functions is still limited. The focus of this study is to provide an overview on isopod crustaceans from the Clarion Clipperton Fracture Zone (CCFZ), an area well-known for its abundance of high-grade polymetallic nodules. Isopods generally comprise an important part of the macrofaunal communities of soft deep-sea sediments and indeed are one of the most dominant macrobenthic groups in the CCFZ. In this review, we have compiled all available data and information on isopod diversity and distribution in the CCFZ in a hybrid manner, which includes published data from the literature as well as the analysis of previously unpublished sources and newly collected data. Although isopods are one of the more prevalent and better-known groups of the CCFZ fauna, this study shows that it is still remarkably difficult to obtain a clear perception of isopod diversity and distribution, as well as the factors that could be responsible for the observed patterns. In many places, knowledge remains incomplete, which is largely due to the low sampling and taxonomic effort, non-standardised sample protocols and the limited taxonomic inter-calibration between studies. The latter is pivotal due to the high proportion of undescribed and presumably new species that typically occur there. An important starting point would therefore be to increase sampling effort and its spatial and temporal coverage in a standardised way, to intensify (integrative) taxonomic work as well as to facilitate sample and data exchange between scientists and contractors. These are fundamental requirements to improve our understanding of the biodiversity of isopods, but also of other faunal groups, in the CCFZ, before mining operations begin.
Similar content being viewed by others
Introduction
Deep-seabed mining
In search of new avenues to tap precious metal and metalloid sources, a number of deep-sea habitats that hold commercially interesting mineral ores were brought into the limelight. The latter include, inter alia, seafloor massive sulphides near hydrothermal vents, cobalt-rich ferromanganese crusts associated with seamounts and abyssal polymetallic nodule fields, which are considered as promising suppliers of many kinds of sought-for metals such as copper, nickel, cobalt, zinc, lead, titanium, lithium, tellurium, silver and gold. However, these mineral deposits are not only of considerable economic value. They also have great ecological and environmental significance as biodiversity reservoirs and for the provision of a range of ecosystem services and functions (Danovaro et al. 2008; Thurber et al. 2014; Fritz 2016). Nodules and crusts increase habitat heterogeneity at multiple spatial scales and concomitantly promote species abundance and richness (Amon et al. 2016; Vanreusel et al. 2016; Gollner et al. 2017). At the same time, they provide important habitats for numerous organisms that may be unique to these areas and are believed to be very susceptible to mining perturbations (Veillette et al. 2007; Schlacher et al. 2014; Gollner et al. 2015, 2017; Singh et al. 2016, 2019; Amon et al. 2016; Vanreusel et al. 2016; Jones et al. 2017). To this end, conservation and exploitation efforts will need to be reconciled to devise environmentally sustainable and economically feasible strategies for seabed mining (Van Dover et al. 2017; Cuvelier et al. 2018; Niner et al. 2018; Jones et al. 2019).
The Clarion Clipperton Fracture Zone (CCFZ) in the eastern tropical Pacific is the primary area of interest for the exploitation of polymetallic (or manganese) nodules. The interplay of different geological, topographical and hydrographic factors and processes has been shown to influence the spatial distribution of polymetallic nodules in the region (Peukert et al. 2018) but also affects community structure and distribution of the sediment and encrusting fauna (Janssen et al. 2015; Vanreusel et al. 2016). On larger spatial scales, gradients in surface productivity and depth across the CCFZ are important drivers of benthic biodiversity and community structure, while small-scale variations in topography and nodule density are likely to explain local patterns (e.g. Bonifacio et al. 2020). Similar environmental gradients were put forward to identify a network of nine large Areas of Particular Environmental Interest (APEIs) bordering the CCFZ to the north and south (Wedding et al. 2013) that are to remain pristine as protected areas and where no influence of mining may occur. The representativeness of these APEIs to fully capture biodiversity and habitats in the region has been under debate (McQuaid et al. 2020; Washburn et al. 2021a), and as a result, four further areas (APEIs #10–13) have recently been adopted into the Environmental Management Plan of the CCFZ (ISA 2021) (Fig. 1). In addition, international regulations set up in the draft “Mining Code” require the establishment of two different types of management zones within exploration contract areas: Impact Reference Zones (IRZ), where mining impacts are to be investigated, and Preservation Reference Zones (PRZ), as control sites where no mining or effects of mining may occur (ISA 2011; Jones et al. 2020).
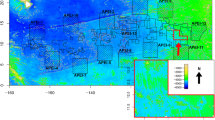
Map of the polymetallic nodule exploration contract areas analysed in this study and Areas of Particular Environmental Interest (APEIs, large squares, APEI#3 and #6 marked in blue and purple, respectively) in the Clarion Clipperton Fracture Zone (CCFZ), superimposed on a map of POC flux at the seafloor (g C m−2 year−1; average 2012–2018; from Bonifacio et al. 2020). In addition, sampled areas from previous studies are shown: DOMES A, Echo1 and PRA. Figure reproduced from Bonifácio et al. (2020) under the Creative Commons license CC BY 4.0
Thus far, the International Seabed Authority (ISA) has issued 17 contracts for the exploration of polymetallic nodules in the CCFZ (as of December 2022; www.isa.org.jm), which are scheduled for a period of 15 years with the possibility of an extension for a further 5 years (Lodge et al. 2014). During this time, contractors are obliged to collect geological, oceanographic and biological information to characterise the baseline status of the ecosystem before mining can occur and additionally to assess the potential impact of mining activities on the marine environment in accordance with ISA regulations (ISA 2000; Lodge et al. 2014). Several exploration contracts have recently been extended for the second 5-year period (December 2021), and the ISA has been put under increasing pressure to adopt the Mining Code for exploitation by July 2023 (e.g. Singh 2021). Therefore, it is imperative to provide detailed baseline information about the resident biota in order to build a comprehensive framework for the environmental management of seabed mining in the CCFZ (Wedding et al. 2013, 2015; Levin et al. 2016; Durden et al. 2017).
Deep-sea Isopoda
Isopod crustaceans are one of the most prevalent and diverse members of deep-sea macrobenthic soft-sediment communities, and due to their ubiquity across deep-sea environments, they are commonly used as target taxa to study variations in abundance, diversity and distribution across a range of temporal and spatial scales (Hessler and Wilson 1983; Rex et al. 1993; Brandt 1995; Wilson 1998, 2017; Stuart et al. 2003; Brandt et al. 2007; Kaiser et al. 2007, 2018; Janssen et al. 2015; Brix et al. 2018, 2020; Raupach et al. 2009). In the CCFZ, isopods also represent one of the most dominant macrofaunal taxa, comprising up to one-third of total macrofauna (Thistle and Wilson 1987, 1996; Kaiser et al. 2015; De Smet et al. 2017; Wilson 2017; Chuar et al. 2020; Pasotti et al. 2021). As with all peracarids, isopods lack planktonic larvae, which imply a restriction of their dispersal capacity. Potentially, this makes obligate brooders, such as isopods, ideal for assessing changes in biodiversity and distributions as a result of environmental disturbances.
In the deep sea, the asellote superfamily Janiroidea Sars, 1897 is particularly successful in both evolutionary and ecological terms, accounting for > 90% of the entire deep-sea Isopoda (Kussakin 1973; Hessler et al. 1979; Hessler and Wilson 1983). Molecular phylogenetic reconstructions indicate an ancient colonisation history of Janiroidea in the deep sea, as evidenced by the likely deep-sea origin of several families (Wilson 1999; Lins et al. 2012; Raupach et al. 2009). Their great success in occupying many different habitats has been attributed to a highly specialised mating system, which is beneficial in low-density environments such as the abyss (Wilson 1991). For most asellote families, detritivory or foraminivory is assumed to be the norm (Svavarsson et al. 1993; Brökeland et al. 2010; Würzberg et al. 2011; Riehl et al. 2018a), but some have been reported to feed on meiofauna (e.g. Munnopsurus spp.; Cartes et al. 2002). Although most species are strictly benthic, following an in- or epifaunal lifestyle, there are transitions towards benthopelagic or even holo-pelagic forms (Osborn 2009).
Thus far, 23 janiroid families—consisting of > 1600 described species—from deep-sea environments have been recorded, with most of these families occurring predominantly or exclusively in the deep sea (Boyko et al. 2008 onwards; Glover et al. 2021). Munnopsidae Lilljeborg, 1864 and Desmosomatidae G. O. Sars, 1897 are typically the most specious and abundant with > 330 and > 120 described species, respectively (WoRMS Editorial Board 2021), and numerous awaiting formal description (Malyutina and Brandt 2018, 2020; Brix et al. 2020, 2021; Jennings et al. 2020). While the majority of janiroidean families and genera appear to be cosmopolitan (with a few exceptions, such as Micromesus Birstein, 1963, Microthambema Birstein, 1961, Pirinectes Malyutina et al., 2020, and Sugoniscus Menzies & George, 1972 thus far only found in Pacific waters, and Parvochelus Brix et al., 2015 as a potential Atlantic endemic), most species seem to have more limited distributions (Brandt et al. 2012). In addition, the large number of suspected new species that are generally found in deep-sea collections (Brandt et al. 2007; Rex and Etter 2010; Kaiser et al. 2017; Golovan et al. 2019) indicates our poor level of knowledge for most deep-sea areas and challenges the evaluation of macroecological patterns of diversity and distributions.
Over the past two decades, advances in deep-sea isopod research have been made due to increased sampling efforts with complimentary sampling devices (Brandt et al. 2016; Kaiser and Brenke 2016) and the use of modern (molecular and modelling) techniques (Brix and Svavarsson 2010; Janssen et al. 2015; Schnurr et al. 2018; Riehl et al. 2018b; Kürzel et al. 2022; Paulus et al. 2022). In particular, application of an integrative taxonomy to derive species boundaries using multiple lines of evidence has led to a more robust identification of species. This, in turn, has facilitated the assessment of diversity patterns and geographic ranges (Brökeland and Raupach 2008; Brix et al. 2011, 2015, 2018; Riehl et al. 2018b; Janssen et al. 2015, 2019; Kaiser et al. 2018, 2021; Schnurr et al. 2018; Riehl and Kühn 2020; Riehl and De Smet 2020). Here, two opposing patterns have emerged; morphologically similar but genetically divergent (i.e. cryptic) species appear to be common (Brix et al. 2015; Janssen et al. 2015; Schnurr et al. 2018; Kaiser et al. 2021), but some isopod species, despite being brooders, are also found to be widespread even surmounting major topographic obstacles (the latter appearing to be the exception and not the rule though, Riehl and Kaiser 2012; Janssen et al. 2015; Bober et al. 2018; Riehl et al. 2018b; Brix et al. 2020; Kaiser et al. 2021). The mechanisms have yet to be clarified, but distance, environmental and topographic barriers as well as adult lifestyle are likely to play an important role in shaping the large-scale distribution of isopod species in the deep sea (Brix and Svavarsson 2010; Bober et al. 2018; Riehl et al. 2018b; Schnurr et al. 2018; Brix et al. 2020; Kaiser et al. 2021).
Study objectives
The aim of the present study is to present a summary of all available data on the diversity and distribution of benthic isopods from the CCFZ. The emphasis is on a literature review of published data, but previously unpublished data will also be considered. We selected three key areas in the study of CCFZ Isopoda and evaluated recent advances in each field. First, we investigate how the abundance and diversity of the isopod fauna is spatially distributed over the CCFZ and discuss the driving factors and processes behind those patterns. We then shift our focus to look at current patterns of species distributions (i.e. endemism and rarity vs. large geographic ranges). We analyse whether evidence from new taxonomic data and molecular techniques can help us to obtain more robust estimates of the geographic distribution and population structure of species in CCFZ Isopoda and to elucidate their primary mechanisms. Finally, we identify gaps in the study of CCFZ isopods in terms of taxonomy, geography or methodology and provide recommendations on how these might be tackled in the future. In the following, we address these questions based on advances in a number of biological disciplines and assess how isopods have helped to understand and answer them. We review the range of data and results from available taxonomic, faunistic and molecular genetic analyses of isopods across the CCFZ to identify gaps in knowledge and determine future research opportunities that will ultimately help to better understand the patterns and processes governing their biodiversity and distribution in the central Pacific nodule province.
Material and methods
Data compilation
We were primarily interested in differences in the abundance, diversity and community composition between different exploration contract areas across the CCFZ. It should be noted here that contract areas cannot be viewed as individual biotopes with specific geographical boundaries. However, for conservation management purposes, for which the data presented here can be used, these artificial limits apply. We extracted all available information on abyssal isopod abundances and occurrences as well as genetic data (COI) from the CCFZ. Some of this information has been published in peer-reviewed journals (published between 1987 and 2021), while a large amount of data is only available in expedition reports or is hitherto entirely unpublished. In addition, the ISA database DeepData (Rabone et al. 2022; https://www.isa.org.jm/deepdata) was queried for isopod occurrences in the CCFZ in order to identify and assess data gaps in the region. Details of the sampling protocols and processing for the unpublished data are provided below.
Sampling and processing
Benthic samples derive from 12 expeditions carried out by the BGR, GSR and IFREMER as well as part of the JPIO MiningImpact and ABYSSLINE projects between 2010 and 2018 (Table S1 in the electronic supplementary). For assessing the abundance and diversity of macrobenthic communities, a box corer and an epibenthic sledge (sensu Brenke 2005) have been deployed (Rühlemann et al. 2010, 2012; Janssen et al. 2013, 2014; Menot et al. 2013; Martínez Arbizu and Haeckel 2015; Uhlenkott et al. 2016, 2017, 2018; Pape et al. 2017, 2018). Here, macrofauna is defined as the size class of animals that pass through a 2000-µm but are retained by a 300-µm mesh. Overall, samples from six contract areas (eastern part of the German contract area [BGR], French [hereinafter referred to as IFREMER], Singapore [OMS], UK [UKSRL, -1B], Belgium [GSR, B4 and B6] and jointly Bulgaria, Cuba, Czech Republic, Poland, Russian Federation and Slovakia as sponsoring states [IOM]) as well as one APEI (#3) were available for the present study (for a summary of the contract areas and station details, see Fig. 1 and Table S1 in the electronic supplementary). In addition, since 2013, two areas for the implementation of a biological time series analysis have been designated in the BGR contract area, one in an established IRZ (PA1-West) and one in a corresponding PRZ located ca. 60 km west of the IRZ. The PRZ was tentatively defined based on a limited dataset that was available at that time and has been sampled regularly since then to test for its suitability as such. For this study, isopod material was available from the PRZ taken by means of an epibenthic sledge in 2013, 2014 and 2015 (MANGAN 2013, 2014 and JPIO MiningImpact SO239 expeditions) and the IRZ taken in 2013, 2014, 2015 and 2016 (MANGAN 2013, 2014, 2016 and JPIO MiningImpact SO239 expeditions, see Table S1 in the supplementary material). Based on this, we were able to assess how the isopod communities have changed over a 3-year time period and whether PRZ and IRZ differed in terms of diversity and composition. The latter gives conclusions as to whether the PRZ is suitable as a preservation area within the BGR contract area. Furthermore, complementary data were available from the DISCOL (DISturbance and reCOLonisation experiment in a manganese nodule area of the deep South Pacific) site, an abyssal manganese nodule area in the Peru Basin, which were collected during the JPIO MiningImpact SO242-1 expedition (Brix et al. 2020; Table S1).
Box corer isopod material that was examined in this study derives from four expeditions: JPIO MiningImpact SO239, MANGAN 2018, GSRNOD15A and GSRNOD17 (Martínez Arbizu and Haeckel 2015; Pape et al. 2017, 2018; Rühlemann et al. 2019; Table S1). All box corer deployments used for these studies had a surface area of 0.25 m2. Unless stated otherwise, sample processing was performed using the following protocol: upon arrival on deck, the surface water above the sediment was removed with a hose and sieved through a 300-µm sieve. A photo was then taken of the box corer surface. Individual biota visible to the eye on the surface (either attached to the nodules or lying on the sediment) were removed and fixed separately (in DESS [Yoder et al. 2006] or 96% ethanol). Nodules were removed and carefully washed to remove surface sediment, measured and weighed for further analyses (analyses not relevant to this study). The sediment in the box corer was then sliced into three layers: 0–3 cm, 3–5 cm and 5–10 cm. Each layer (0–3 cm, 3–5 cm and 5–10 cm) was transferred into cold filtered sea water (2–4 °C), carefully elutriated and separately sieved (through a 300-µm mesh) and fixed. During MANGAN 2018, one additional subcore was taken from each box corer for sedimentological assessment prior to slicing (Rühlemann et al. 2019). During SO239 (Martínez Arbizu and Haeckel 2015), the top layer (0–3 cm) was immediately sorted, and specimens were separately fixed (80 and 96% undenatured ethanol). The remaining layers were fixed in 4% formalin and later transferred to 80% denatured ethanol. During GSRNOD15A (Pape et al. 2016), the top 0–3-cm layer was fixed in bulk in 96% undenatured EtOH at − 20 °C; the deeper layers were first stored for 48 h in 10% formaldehyde, after which these were transferred to 80% undenatured EtOH. During GSRNOD17 (Pape et al. 2018), all sediment layers were stored in 96% undenatured EtOH at − 20 °C. Sorting of the box corer material was partly conducted on board, but mainly back in the home laboratories (IFREMER in Plouzané [France], Senckenberg am Meer—DZMB in Wilhelmshaven [Germany], or the Marine Biology Research Group Laboratory facilities in Ghent [Belgium]).
A detailed description of the epibenthic sledge can be found in Brenke (2005). In short, it consists of two superimposed nets, i.e. a lower epinet and an upper supranet, each with a mesh size of 500 µm, and ending in 300-µm cod ends. A door, attached to an opening/closing mechanism, is located in front of the net, which remains closed in the water column and opens as soon as the epibenthic sledge touches the sea floor. In this way, contamination by planktonic organisms can be minimised. For sampling in warm, tropical nodule areas, an additional metal grid is placed in front of the mesh openings to prevent nodules from entering the nets and thus damaging the samples. Furthermore, a box is placed around the cod ends, which holds cold deep water. This is to ensure a continuous cooling chain, which is essential for genetic analyses (Riehl et al. 2014a). For JPIO MiningImpact SO239 deployments, the trawling distance (d) was calculated in order to enable standardisation of the trawled seabed to 1000 m and thus assessment of faunal densities (ind. per 1000 m2), as the epibenthic sledge sample unit is 1-m wide (Table S1). Therefore, the following formula was used:
where V1 is ship velocity during trawling, T1 is trawling time, V2 is ship velocity during haul, T2 is haul time (sledge off bottom), V3 is winch velocity and T3 is haul time (sledge off bottom).
For all expeditions, sample processing followed a standard procedure. As soon as the epibenthic sledge was back on board, the nets were rinsed with sea water up to the cod. The cod ends of both nets were removed and immediately transferred to a cold room (mostly at ~ 2–4 °C and 11 °C during BIONOD). Epi- and supranet samples were processed separately. The samples were elutriated with filtered pre-cooled sea water, and the macrofauna was placed on a 300-µm mesh. Each sample was preserved in 96% pre-cooled undenatured ethanol and kept at low temperature (− 20 °C) for at least 48 h (Riehl et al. 2014a). Macrofauna samples were then sorted on board and back at DZMB using stereomicroscopes to a higher taxon level (phylum, class, order) and stored in 96% undenatured ethanol. Isopod individuals were only counted if the head was present. Isopod samples were usually further discriminated to family level and partly to morpho-species level. If species could be morphologically assigned to known species, species names were used, while presumably new species were assigned a unique numerical code. Isopod specimens that were too damaged to be assigned to a species were marked as sp. indet. All isopod specimens that were not used for taxonomic descriptions are deposited with the DZMB in Wilhelmshaven or at the Marine Biology Research Group of Ghent University. Type material of described species is either stored at the Senckenberg Museum, Frankfurt (SMF), or the Crustacean Collection of the Museum of Nature (Leibniz Institute for the Analysis of Biodiversity Change, LIB) (formerly Center of Natural History, CeNak) in Hamburg (Kaiser et al. 2018, 2021; Malyutina et al. 2020, see Table S2 in the electronic supplementary for accession numbers).
Since data collected by box corer and epibenthic sledge are not entirely comparable (Lins and Brandt 2020) for assessing diversity and community composition, we only report on data collected by means of the epibenthic sledge for these parameters. Isopod specimens from the box corer of the MANGAN 2018 and GSR expeditions were used for genetic analysis exclusively to assess species’ geographic range size and diversity at the regional scale.
DNA extraction, amplification, sequencing and molecular species delimitation
Published COI sequences were taken from GenBank (Table S2; Janssen et al. 2015, 2019; Kaiser et al. 2018, 2021; Riehl and De Smet 2020; Brix et al. 2020). Additional COI sequences were obtained from 486 isopod individuals summing up to a total of COI sequences from 1522 (epibenthic sledge: 1471; box corer: 51) individuals (Table S2). From each specimen, 2–3 legs were dissected unilaterally to keep the voucher specimen intact for later morphological re-assessment. All tissue samples were initially stored in 96% undenatured ethanol. Prior to extraction, all tissue samples were washed in distilled water to remove excess ethanol. DNA was then extracted using Chelex® 100 BioRad (Walsh et al. 1991) based on the protocol provided by Janssen et al. (2015). DNA from isopods from the GSR expeditions (GSRNOD15A and GSRNOD17, Table S2) was extracted using the NucleoSpin Tissue XS kit (Macherey–Nagel, Germany). Amplification of the COI barcode was conducted using universal primers introduced by forward: LCO1490 5′-ggtcaacaaatcataaagatattgg-3′ and reverse: HCO2198 5′-taaacttcagggtgaccaaaaaatca-3′ (Folmer et al. 1994) following previously published protocols (sensu Janssen et al. 2015). PCR products that produced light bands after electrophoresis on 1% agarose gel were sent to the MacroGen Europe Laboratory in Amsterdam, Netherlands, for sequencing using the same set of primers as used for the PCR. DNA from isopods of the JPIO MiningImpact SO239 and JPIO MiningImpact SO242-1 expeditions were extracted using the PCR protocols of Brix et al. (2011) and Riehl et al. (2014a). Extraction and sequencing of the individuals was conducted at the Laboratories of Analytical Biology (LAB), Smithsonian National Museum of Natural History, Washington DC, USA, with an ABI 3730xl 96-well capillary sequencer. A consensus sequence was produced for every individual, combining reverse and forward sequences using SeqTrace (Stucky 2012) or Geneious Prime 2020. The consensus sequences were aligned in the MEGA6 (Tamura et al. 2013) and Geneious 10.1.2 (MAFFT alignment) softwares.
To assess the number of species (or rather MOTUs) in the COI dataset, a number of species delimitation (SD) analyses were conducted. Multiple delimitation methods were employed on the COI dataset to access potential congruence (Carstens et al. 2013). Four different distance- and tree-based species delimitation analyses were performed to allocate sequences into genetic species. Distance-based approaches (Assemble Species by Automatic Portioning, ASAP: Puillandre et al. 2021; Barcode Index Number, BIN: Ratnasingham and Hebert 2013) detect the distance at which the “barcode gap” occurs and sort the sequences into putative species based on this distance. ASAP analysis was implemented on the web interface (https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html) (Puillandre et al. 2021) with default setting and under the p-distance model. Barcode Index Numbers (BINs) were assigned on the registered DNA dataset automatically using the BOLD v.4 workbench (www.boldsystems.org). Tree-based approaches (multi-rate Poisson Tree Processes, mPTP: Kapli et al. 2017; General Mixed Yule Coalescent, GMYC: Pons et al. 2006) use a phylogenetic tree from which the fit of speciation and coalescent processes are modelled to delineate species based on the branching rate of the tree (Carstens et al. 2013; Tang et al. 2014). The ultrametric phylogenetic tree was produced in BEAST v.2.5. Settings were as follows: strict clock, Yule speciation model, two independent MCMC chain runs for 20,000,000 generations, and sampled every 1000 steps (10% was discarded as burn-in period). The best-fitting model of nucleotide substitution was selected using jModelTest v.2.1.10 for each family separately under the Akaike information criterion (Posada 2008). The model selected for COI was either HKY + I + G (Dendrotionidae, Haplomunnidae, Mesosignidae) or GTR + I + G (Desmosomatidae, Haploniscidae, Ischnomesidae, Macrostylidae, Munnopsidae, Nannoniscidae). Convergence of BEAST runs was assessed with Tracer v1.7.1 (Rambaut et al. 2018) and burn-in was selected after all effective sample sizes (ESSs) were at least 200. The GMYC method was implemented using the R package SPLITS (Fujisawa and Barraclough 2013), under the single-threshold model (stGMYC), while the mPTP was implemented on the web server (https://mptp.h-its.org) using the multi-rate Poisson tree process model and following default settings.
Community and diversity analysis
Due to the heterogeneity of the data, we kept box corer and epibenthic sledge data separate and reduced the datasets to the lowest common taxonomic denominator in order to enable a comparison between the different studies. Family-level data were available for a number of epibenthic sledge samples (Table S3). In some cases, data from epi- and supranet were merged (SO205, 2014, SO239); in other cases, only supranet data were available (BIONOD, SO239 stations #192 and #197; Table S3). Since it is assumed that epi- and supranet have similar faunal compositions (e.g. Knox et al. 2012), a family station matrix was created for the community analysis based on relative abundances (%) including both merged (epi and supra) and supranet-only samples (Table S3). In addition, for the SO239 dataset, family-level data were available from both epibenthic sledge and box corer (Tables S3, S4), so a direct comparison in family composition between the two devices could be made. Since most collected species were putatively new to science, and taxonomic calibration exercises have not yet been achieved, species-level analyses were conducted using the number of MOTUs per area (Table S2).
To compare MOTU richness between different contract areas within the CCFZ, APEI#3 and DISCOL, as well as PRZ and IRZ within the BGR contract area (as part of the time series), individual-based rarefaction curves were computed in PAST version 3.26 (Hammer et al. 2001), as these avoid bias caused by different sample sizes (Gotelli and Colwell 2001). In addition, abundance-based (Chao 1) and incidence-based (Chao 2, Jackknife 1 and 2 and Bootstrap) richness estimators were calculated in PAST to predict total richness per predefined area (Magurran 2004). Therefore, samples for each area (BGR, UKSRL, OMS, IOM, GSR, IFREMER, APEI#3, DISCOL) were pooled prior to analysis. Chao 2, Jackknife 1 and 2 and Bootstrap were only computed for all contract areas (BGR, UKSRL, OMS, IOM, GSR, IFREMER) combined to extrapolate isopod richness for the CCFZ as a whole.
To assess the spatial and temporal variation in isopod composition between different areas, four matrices were analysed based on (1) a family × station matrix for relative abundance (percentage) per family based on epibenthic sledge samples from across the CCFZ, (2) a family × station matrix for relative abundance (percentage) per family based on box corer and epibenthic sledge samples from the SO239 expedition, (3) a MOTU × area (including contract areas, APEI3 and DISCOL) matrix based on relative abundance per MOTU and (4) a MOTU × area (IRZ and PRZ within the BGR contract area) matrix based on relative abundance per MOTU. A nonmetric multidimensional scaling (nMDS) based on a Bray–Curtis similarity matrix was plotted to visualise differences between locations using Primer 6.0. A one-way ANOSIM was computed in Primer 6.0 to test for significant differences in family and MOTU composition between different areas.
Results and discussion
A brief history of isopod research in the CCFZ
Initial investigations of the CCFZ isopod fauna date back to US NOAA funded programs conducted between 1970 and 1992, with data collected from different sites—DOMES A, Echo 1 and PRA—in the central and western CCFZ (Thistle and Wilson 1987, 1996; Wilson 1990, 2017; Fig. 1). With the resumption of interest in seabed mining during the last two decades, a larger spatial spectrum of information for isopods from nine different contract areas in the CCFZ, as well as first collections from APEIs (#3, #6 and #9), has become available (Janssen et al. 2015, 2019; Kaiser et al. 2015, 2021; De Smet et al. 2017; Yu et al. 2018; Brix et al. 2020; Chuar et al. 2020; Pasotti et al. 2021; Washburn et al. 2021a,b; summarised in Table 1). In addition, the first genetic data from isopods were obtained from the CCFZ (Janssen et al. 2015, 2019; Brix et al. 2020; Riehl and De Smet 2020; Kaiser et al. 2018, 2021).
Despite the wealth of information that has been gathered over the years, comparison between studies is challenged by the fact that different sampling devices have been used for data collection and that much of these data are only available as grey literature (e.g. Wilson 1992; Smith et al. 2008; Martínez Arbizu and Haeckel 2015). Isopod records extracted from the ISA DeepData database indicate that most of the isopod material derives from sampling with epibenthic sledge, multicorer and box corer (Fig. 2), but physical specimens and/or images were also collected, albeit to a much lesser extent, by ROV (e.g. Martínez Arbizu and Haeckel 2015). The use of box corer, as a quantitative tool, was recommended for macrofauna sampling by the Legal and Technical Commission of the ISA (ISBA/25/LTC/6Rev1; ISA 2020). As one box corer with a standard size of 50 × 50 cm usually has very low macrofaunal densities (e.g. Washburn et al. 2021b), the epibenthic sledge was introduced as an additional type of gear during the sampling campaigns of the BGR (e.g. Rühlemann et al. 2010, 2012, 2019), in the course of JPIO MiningImpact (Martínez Arbizu and Haeckel 2015), and later by UKSRL as part of the ABYSSLINE project (Dahlgren et al. 2016). The epibenthic sledge is more suitable to sample larger amounts of epi- and suprabenthic macrofaunal specimens compared to the box corer, even if only in a semi-quantitative manner (Kaiser and Brenke 2016; Lins et al. 2021).
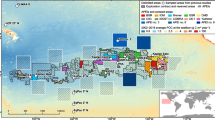
Summary of the CCFZ isopod datasets (n = 3367 total) in the ISA DeepData database: a proportionally per family (note: ca. 80% of samples in the database were not identified to family level); b per contractor area and c per gear type. BC, box corer; EBS, epibenthic sledge; MUC, multicorer; BGR, eastern German contract area; GSR, Global Sea Mineral Resources NV (Belgian contract area); IFREMER, L’Institut Français de Recherche pour l’Exploitation de la Mer (French contract area); IOM, Interoceanmetal Joint Organization contract area; OMS, Ocean Mineral Singapore Pte. Ltd. (Singapore contract area); UKSRL, UK Seabed Resources Ltd. (UK contract areas); KR, contract area of the Korean Republic; DORD, Deep Ocean Resources Development Co. Ltd. (Japanese contract area); YUZM, JSC Yuzhmorgeologiya (contract area of the Russian federation)
Box corer and epibenthic sledge are known to sample different numbers and fractions of the fauna, and thus to result in different patterns of abundance, diversity and composition that are only comparable to a limited extent (e.g. Jóźwiak et al. 2020; Lins and Brandt 2020). In addition, even for one type of sampling gear such as the box corer, different sampling protocols and mesh sizes (250 vs. 300 µm) were used for the various studies (Table 1). This influences the sampling efficiency and thus, in turn, the comparability of the diversity and abundance of fauna between studies (e.g. De Smet et al. 2017; Yu et al. 2018; Chuar et al. 2020; Washburn et al. 2021b). Last but not least, differences in taxonomic resolution (family vs. species level) between studies derived from morphological and/or molecular data as well as considering the sample set as a whole or only subsets of the data have provided a rather mixed picture of the isopod biodiversity in the CCFZ (see also Table 1).
Species delimitation
Reliable species delimitation is vital in order to be able to estimate the diversity of species, their distribution and ultimately the levels of impact from mining operations. In particular, the question of whether there are few species with a wide spatial distribution range or many species with only a limited range would have major implications for conservation strategies, including questions on the size of preservation areas and their distances from mining blocks.
Isopod species from the abyssal CCFZ were initially solely delimited by morphological means (Thistle and Wilson 1987, 1996; Wilson 1990, 2017; Malyutina 2011; Kaiser 2014; Riehl et al. 2014b; Malyutina et al. 2020). However, as in many other taxa, the morphological delineation of isopod species is complicated by the presence of morphologically very similar or identical, but genetically distinct species (Pfenninger and Schwenk 2007; Janssen et al. 2015; Kaiser et al. 2021). Also known from several families are a number of sexually strongly dimorphic species in which males are difficult to assign to the corresponding females (Riehl et al. 2012; Paulus et al. 2022). Small-sized crustaceans such as isopods are also prone to misidentification as they can easily lose their legs during sampling or sample processing. Since legs often bear taxonomically important characters, individuals in this case could possibly not be assigned to a species or even genus level (Frutos et al. 2022). Therefore, an integrative approach that links multiple lines of evidence to derive species boundaries is now increasingly being pursued (Carstens et al. 2013; Kaiser et al. 2018, 2021; Brix et al. 2020; Riehl and De Smet 2020). For the CCFZ, the study by Janssen et al. (2015) was the first to combine mitochondrial DNA sequences (COI) and morphological data. Their results revealed a sometimes-large discrepancy between morphological and genetic diversity, with the number of MOTUs being two to three times higher than the number of morphological species (Janssen et al. 2015).
In our study, we defined MOTUs on the basis that the majority of SD methods applied were congruent. In addition, in most cases, molecular delimitation was backed up by additional morphological examination. For most species, this approach was unequivocal, i.e. all SD methods showed the same species demarcations, but some also gave incongruent results (Table S2 in the electronic supplementary). The latter could be cases where the genetic distances between clades fall within the barcode gap, as previously shown for CCFZ isopods (Kaiser et al. 2018, 2021). Overall, SD methods seem to behave differently, especially when looking at single-locus data (Dellicour and Flot 2018), as presented here, with genetic diversity, effective population size and speciation rates negatively affecting their performance (Dellicour and Flot 2018; Magoga et al. 2021). Tree-based approaches like GMYC tend to overestimate true species numbers, whereas distance-based approaches like Automatic Barcoding Gap Discovery (ABGD) or ASAP tend to lump species together and thus underestimate species richness (Dellicour and Flot 2018; Kaiser et al. 2018, 2021; Paulus et al. 2022). Therefore, the aim should be to use several SD methods in combination, the agreement of which then implies a correct species delimitation (Dellicour and Flot 2018).
Based on the above considerations, we assume that our differentiation is robust by combining different SD methods and linking them to morphology. On this basis, we could delineate 361 MOTUs, 345 of which were collected with the epibenthic sledge and 16 additional obtained from the box corer. Only eighteen MOTUs could be assigned to known species (e.g. Acanthocope galatheae Wolff, 1962, Rectisura slavai Malyutina, 2011, Table S2), while the majority appear to represent species new to science (~ 95%). Notably, seven of these species and two genera (Ketosoma Kaiser & Brix, 2018; Pirinectes Malyutina & Brix, 2020) have been newly described from this dataset (Kaiser et al. 2018; Malyutina et al. 2020; Riehl and De Smet 2020; Kaiser et al. 2021; Table 2). The wide distribution of Acanthocope galatheae, a species described from the Gulf of Panama and also distributed in the Atlantic Ocean, has been molecularly confirmed (S. Bober, pers. comm.). Others, such as Betamorpha fusiformis (Barnard, 1920), occurring out of their presently known range, would need further examination. For the latter, a species complex has been identified that spans the Southern Ocean and the Atlantic (Raupach et al. 2007), making it very likely that the species occurring in the CCFZ represents a different and potentially new species. Our SD analysis also suggests that some species, in particular Macrostylis metallicola Riehl & De Smet, 2020 and Rectisura slavai, probably represent more than one species (Table S2). However, further analyses are required to confirm these findings.
The addition of molecular (barcoding) methods to morphology-based taxonomy has certainly helped advance the differentiation of isopod species, but the latter remains complex and challenging. The integrative approach, considering multiple lines of evidence, including morphological, molecular, ecological and/or biogeographical data, currently seems to be the best way forward to provide a stable taxonomy but requires significant time, financial resources and above all strong taxonomic expertise. With deep-seabed mining becoming imminent, however, methods must be found to assess and compare species richness and distribution more rapidly, while ensuring reliable species assignment. These include, on the one hand, the use of modern molecular methods such as proteomic fingerprinting, which has been successfully tested as a rather cheap and fast method for species delimitation of deep-sea isopods (Kürzel et al. 2022; Paulus et al. 2022). On the other hand, trained taxonomists are needed to identify and describe the many unnamed or new species, but also to produce identification keys and train future taxonomists to ensure thorough biodiversity assessment and monitoring in the CCFZ in the long term.
Spatial structure of isopod composition, abundance and diversity across the CCFZ
Composition
In many ways, macrobenthic communities of the CCFZ resemble other (nodule-free) abyssal areas, at least, when looking at supra-specific taxa, as similar groups exist and polychaetes and isopods typically dominate samples (e.g. Hessler and Jumars 1974; De Smet et al. 2017; Gollner et al. 2017). This is also reflected in isopods at the family level; CCFZ isopod communities mainly consist of typical deep-sea families (e.g. Munnopsidae, Desmosomatidae, Haploniscidae, Nannoniscidae, Macrostylidae; Fig. 3, Tables S3, S4), which is similar to ocean basins elsewhere (e.g. Brandt et al. 2007; Lörz et al. 2013; Elsner et al. 2015). Yet, there are profound differences in isopod family composition between box corer and epibenthic sledge collections. The families Munnopsidae and Desmosomatidae are the most dominant families in epibenthic sledge samples, whereas Nannoniscidae, Macrostylidae and Thambematidae, representing more infaunal taxa, are more prevalent in box corer samples (Figs. 4 and 5, see also Thistle and Wilson 1996; Janssen et al. 2015; De Smet et al. 2017; Yu et al. 2018; Brix et al. 2020; Pasotti et al. 2021). In addition, typically fewer families are found in box corer samples compared to the epibenthic sledge (Fig. 4). On the one hand, this could be related to a gear-dependent bias, in which epibenthic taxa are swept away by a large bow-wave effect when the box corer hits the seafloor (e.g. Lampitt et al. 1986; Pasotti et al. 2021). On the other hand, the patchy distribution of many deep-sea families may play a role, while others are only rarely encountered overall (e.g. families Janirellidae or Katianiridae, cf. Kaiser et al. 2007). As the sample size from the box corer is much larger than that of the epibenthic sledge, many individuals are simply not collected. Thus, since epibenthic sledge and box corer collect species with different sediment associations (more epifaunal vs. more infaunal), together with the above-mentioned sampling specificities of the individual devices, the requirement for simultaneous use of both arises in order to record the diversity of the CCFZ (isopod) fauna more completely.
Representative isopod families found in CCFZ collections: a Desmosomatidae, Prochelator sp. stet.; b Mesosignidae, Mesosignum sp. stet.; c Dendrotionidae, Dendrotion sp. stet.; d Janirellidae sp. stet.; e Haploniscidae sp. stet.; f family incertae sedis, Sugoniscus cf. parasitus Menzies & George, 1972; g Munnopsidae, Eurycope sp. stet.; h Nannoniscidae, Nannoniscus pedro Kaiser, Brix & Kihara, 2021; i Nannoniscidae, Ketosoma ruehlemanni Kaiser & Janssen, 2018; j Macrostylidae, Macrostylis sp. stet. Images courtesy of A. Janssen, S. Kaiser; B. Wawrzyniak-Wydrowska
Relative abundances of isopods in epibenthic sledge vs. box corer samples; samples were collected during the JPIO MiningImpact SO239 expedition (Martínez Arbizu and Haeckel 2015). APEI, Area of Particular Environmental Interest; BGR, eastern German contract area; GSR, Global Sea Mineral Resources NV (Belgian contract area); IFREMER, L’Institut Français de Recherche pour l’Exploitation de la Mer (French contract area); IOM, Interoceanmetal Joint Organization contract area. Corresponding raw data in Tables S3 and S4 in the electronic supplement
Nonmetric multidimensional scaling (nMDS) ordination based on a Bray–Curtis resemblance matrix using relative abundances (%), showing differences in isopod family composition between epibenthic sledge (circles) and box corer (asterisks) samples collected during the SO239 expedition. Red: BGR; orange: IOM; green: GSR; turquoise: IFREMER; blue: APEI#3. For interpretation of the references to colour in this figure legend, the reader is referred to the online version of this article
Remarkably, there is a discrepancy between the family composition of the analysed samples and the number or datasets per family registered in the DeepData database, where most of the records were assigned to the Desmosomatidae, whereas the Munnopsidae are only rarely represented (Figs. 2a and 4). In any case, the database only contains data discriminated to the family level for a relatively small proportion of the isopods (~ 20%). In addition, the data are geographically very unevenly distributed. By far, the majority of the data comes from the eastern contract areas, such as BGR, whereas hardly any data are available from more westerly located sites (Fig. 2b), which could distort perceived patterns and their interpretation.
Chuar et al. (2020) analysed family-level composition in box corer samples within the OMS contract area and found that family composition of isopods differs between stations. Given the small spatial scale of the study, this finding is quite remarkable. We examined the composition of isopod families collected by the epibenthic sledge from a much larger geographic area spanning four contract areas and APEI#3 (Fig. 6). The analysis of these data also revealed a significant variation in family composition (one-way ANOSIM; R: 0.284, p = 0.0182, n permutations: 999), but the low global R-value suggests a high faunal overlap between areas. In fact, significant differences could only be demonstrated between the BGR contract area and APEI#3, while there were no differences in family composition between the other areas (Fig. 6). This lack of spatial differentiation at the family level is not uncommon; many isopod families found in the abyss have broad geographic distributions and are found “everywhere”, albeit in varying proportions (Brandt et al. 2007; Meyer-Löbbecke et al. 2014; Elsner et al. 2015). In addition, most isopods collected from the abyssal CCFZ consume food items of low nutritional value, so that a distinct spatial structure, such as different distribution patterns of guilds (e.g. carnivorous vs. deposit feeding families) linked to regionally different inputs of particulate organic carbon (POC) across the CCFZ, as seen in polychaetes (e.g. Bonifacio et al. 2020; Washburn et al. 2021b), was not expected for isopods. However, it is possible that as geographic scale increases, such as when comparing different ocean basins, we may see a distinct CCFZ community at the family level (Kaiser et al. unpubl. data, see also Singh et al. 2016 for meiofaunal Nematoda).
Nonmetric multidimensional scaling (nMDS) ordination based on a Bray–Curtis resemblance matrix using relative abundances (%), illustrating similarity of isopod family composition between different CCFZ contract areas and APEI#3 (data from SO205, BIONOD, MANGAN 2014 and SO239 expeditions: S. Schnurr, unpubl. data, Kaiser et al. 2015, unpubl. data). Red: BGR; orange: IOM; green: GSR; turquoise: IFREMER; blue: APEI#3. For interpretation of the references to colour in this figure legend, the reader is referred to the online version of this article
At enhanced taxonomic resolution, differences between communities became more apparent. Brix et al. (2020) analysed the MOTU composition of four dominant isopod families (Munnopsidae, Desmosomatidae, Macrostylidae and Haploniscidae) in the CCFZ, APEI#3 and the DISCOL area, with their data representing a subset of our dataset. Based on relative abundance data, they found CCFZ contract areas to be more similar to each other than to APEI#3 or the distantly located DISCOL area (Brix et al. 2020). On the other hand, presence/absence of data revealed high dissimilarity also between CCFZ sites, driven by the high proportion of unique site-endemic species (Brix et al. 2020). Notably, more species were shared between CCFZ contract areas and the DISCOL area more than 5000 km away than with the much closer APEI#3.
The analysis presented here is an extension of datasets from Janssen et al. (2015) and Brix et al. (2020). Overall, there appears to be a decay in isopod similarity with increasing distance, i.e. we found greater similarity between UK-1, OMS, BGR, IOM and GSR contract areas clustering more closely together, while APEI#3 and DISCOL were more dissimilar and the IFREMER contract area taking an intermediate position (Fig. 7a). Low faunal similarity (0–7.7% Bray–Curtis similarity) between APEI#3 and the contract areas indicates that APEI#3 is not fully representative for the isopod biodiversity in the CCFZ—at least based on the current results and for the contract areas studied. This is consistent with patterns in other taxa suggesting that the faunal composition of APEIs is different from that of CCFZ contract areas (Taboada et al. 2018; Błażewicz et al. 2019; Bonifacio et al. 2020; Brix et al. 2020; Christodoulou et al. 2020; Washburn et al. 2021a, b). This generally casts doubt on the representativeness of the APEIs studied so far as potential faunal sanctuaries for recolonisation. Analyses of the newly defined APEIs will be required to test whether they are more representative of the fauna in the potential mining areas of the CCFZ.
Nonmetric multidimensional scaling (nMDS) ordination (a) and hierarchical cluster analysis (b) of transformed (relative abundance) epibenthic sledge data based on a Bray–Curtis similarity matrix for CCFZ isopods at the MOTU level. Red: BGR; yellow: UK-1; grey: OMS; orange: IOM; green: GSR; turquoise: IFREMER; blue: APEI#3; black: DISCOL
Last but not least, we were able to analyse differences in the MOTU composition between two different areas (PRZ and IRZ) within the BGR contract area. These data allowed us to examine changes in the community over a 3-year period. In addition, valuable data on the representativeness of the PRZ as a protection zone within a contract area could be collected. The results of our time series analysis showed significant differences between IRZ and PRZ (one-way ANOSIM; R: 0.889, p = 0.029). However, it also became clear that the communities in each area were not static but varied over the study period (Fig. 8). From this, changes in the sampling strategy can be derived, such as the spatial and temporal intervals of sampling, which will be discussed further below. The strong differences we observed between the IRZ and the PRZ suggest that while the PRZ isopod fauna does partly overlap with the IRZ fauna (i.e. a number of species are shared by both areas), it does not suit its function as a similar control site. It would also not fulfil a complete protective function for the potential recolonisation of the impacted mining site.
Nonmetric multidimensional scaling (nMDS) ordination for CCFZ isopod assemblages based on a Bray–Curtis similarity (relative abundance) matrix, reflecting differences in isopod MOTU composition between the BGR Preservation Reference Zone (PRZ) and Impact Reference Zone (IRZ) over a period of 3 years (2013–2016). For PRZ, no sequences are available from 2016
Abundance
Large-scale comparisons of isopod densities (ind. per m2) obtained with box corer show large differences between and within contract areas (Fig. 9). However, there is a slight decline in densities from more easterly to more westerly located contract areas across the CCFZ (De Smet et al. 2017; Wilson 2017; Chuar et al. 2020; Fig. 9), which appears to be linked to changes in primary productivity (Washburn et al. 2021b). This is the case with other taxa as well (Glover et al. 2002; Wilson 2017; De Smet et al. 2017; Bonifacio et al. 2020; Washburn et al. 2021b). For instance, isopod densities in the OMS contract area in the eastern part of the CCFZ are in the range of 78.91 ± 42.89 (mean ± SD), compared to 27.02 (mean) ind. per m2 at DOMES A in the western part of the CCFZ (Wilson 2017; Chuar et al. 2020). PRA sites in the central part of the CCFZ, with 82.25 isopods ind. per m2 (mean), show values similar to those of the eastern OMS contract area, but significantly higher values than the central Echo 1 (41.07 ind. per m2) and GSR (15–18 ind. per m2) areas. This could be associated with a temporarily higher POC flux during the time of sampling (De Smet et al. 2017; Wilson 2017; Chuar et al. 2020; Fig. 9). It should be noted that there is not enough information from the western CCFZ, and more data should be collected in order to adequately assess density differences along latitudinal and longitudinal gradients (see also Washburn et al. 2021b).
In addition to food availability, a number of other environmental parameters could play a role in determining the abundance of isopods in the CCFZ. In contrast to some other taxa (e.g. sessile megafauna, Vanreusel et al. 2016), however, no clear relationship could so far be established between isopod and nodule densities (Pasotti et al. 2021; Washburn et al. 2021b). Washburn et al. (2021b) argued that the weak relationship between nodule and isopod densities in their study could be due to the use of regional models for nodule abundance estimation; nodule densities in the CCFZ are quite heterogeneous and can vary greatly within a few tens to hundreds of metres (Peukert et al. 2018). This small-scale variation has not been included in the models and therefore may not correspond to the degree of biological variation (Washburn et al. 2021b). In the study by Pasotti et al. (2021), however, nodule abundance was derived from in situ box corer collections and could therefore be directly related to isopod densities. The lack of a significant relationship between these two variables implies that it is arguably not an artefact.
Depth differences have often proven to be an important factor determining macrofaunal abundances in the deep sea (e.g. Rex et al. 2006), and thus, depth-related factors may also explain the variation in isopod densities in the CCFZ. From the eastern (e.g. BGR, UKSRL, OMS) to the more centrally located contract areas (e.g. IFREMER), depth gradually increases by over 1000 m, and this difference in depth appears, if only slightly, to be reflected in isopod abundances (Washburn et al. 2021b). Yet, because depth changes along a longitudinal gradient as does surface productivity, it can be difficult to disentangle these effects from one another.
Overall, it has become clear that the influence of environmental factors on faunal communities is largely masked by sampling effects. Washburn et al. (2021b), for instance, analysed a large dataset consisting of samples from > 400 box corers across the CCFZ and found that ~ 36% of the variation in isopod abundance was explained by “random” study-related effects. Thus, differences in sampling efficiency due to the use of different sampling protocols among studies can also have a profound impact on the observed abundance patterns.
Diversity
Patterns of local and regional isopod diversity have been investigated by morphological and molecular means (Thistle and Wilson 1996; Janssen et al. 2015; De Smet et al. 2017; Wilson 2017; Brix et al. 2020; Pasotti et al. 2021; Washburn et al. 2021b). The main objectives of these studies were to compare isopod diversity between different contract areas and APEIs, to identify the primary factors influencing diversity, as well as to assess the representativeness of diversity data at the regional scale (Janssen et al. 2015; De Smet et al. 2017; Brix et al. 2020; Washburn et al. 2021b). As described in the previous section, the comparison of isopod diversity between studies is hampered by differences in existing sampling equipment and protocols (see also Washburn et al. 2021b). Other problems emerged from the use of different (molecular vs. morphological) approaches; as previously mentioned, morphological assessment tends to underestimate actual species richness (cf. Janssen et al. 2015), while the use of molecular mitochondrial markers typically overestimates species numbers (Song et al. 2008; Dietz et al. 2015; Ribardière et al. 2017). Finally, a meaningful regional comparison can only be made when all species accumulation curves have attained an asymptote (Gotelli and Colwell 2001), which has not yet been achieved for CCFZ isopods in any study (De Smet et al. 2017; Wilson 2017; Brix et al. 2020; Pasotti et al. 2021; Washburn et al. 2021b).
Even within contract areas, local isopod diversity can show large variation. For example, Thistle and Wilson (1996) reported that local richness of isopods from box corer samples in the central part of the CCFZ (Echo 1) varied between 2 and 20 species (mean ± SD; 8.4 ± 5.0 species; 15 box corer deployments). Isopod richness in the close-by GSR area appears to be considerably lower, ranging between 2.00 ± 0.01 and 3.7 ± 0.7 isopod species per box corer (De Smet et al. 2017; Pasotti et al. 2021). Our time series data from the BGR contract area confirm the high spatial but also temporal variation in isopod diversity. That is, we found large differences in diversity between relatively close areas within the BGR contract area (PRZ and IRZ), as well as strong temporal variability within each area (Fig. 10). Differences in sediment parameters and nodule coverage may play a role in explaining spatial diversity variation at the local scale. Yu et al. (2018), analysing macrofauna (incl. isopods) from the contract area of the Korean Republic, for example, found higher diversity to be linked to higher nodule coverage. By contrast, Pasotti et al. (2021) tested the effect of nodule densities on local diversity patterns and did not find a significant relationship. Episodic food input from surface waters and changes in hydrographic conditions (e.g. erosive currents, cf. Thistle and Wilson 1987, 1996; Levin et al. 2001) are probably explanatory parameters for temporal differences in diversity. Overall, it has been shown that abyssal areas can be very dynamic on relatively small spatial and temporal scales (Kaiser et al. 2007; Kuhnz et al. 2014; Taylor et al. 2017). However, better understanding of natural spatial and temporal trends is important to distinguish background variability from the impact of deep-sea mining (Amon et al. 2022). One-off or short-term studies are therefore not sufficient to capture the natural variability of abyssal communities (e.g. Kuhnz et al. 2014) and this should be considered in future investigations.
To date, the studies by Janssen et al. (2015), Wilson (2017), Brix et al. (2020) and Washburn et al. (2021b) are the most spatially extensive (i.e. comparing isopod diversity between different nodule areas). Janssen et al. (2015) and Brix et al. (2020) investigated differences in isopod diversity based on epibenthic sledge samples using an integrative (morphological/molecular) approach, while the data in Wilson (2017) and Washburn et al. (2021b) derive from box corers and relate to the morpho-species level. Assessing differences in isopod and polychaete diversity between the eastern BGR and the IFREMER contract area was one focus of the study by Janssen et al. (2015). In total, they could distinguish 95 MOTUs within seven isopod families, whereby significant differences between the BGR and IFREMER contract areas did not become apparent. Brix et al. (2020) looked at only four isopod families but from a much wider geographic area. In their study, a total of 187 species from 22 epibenthic sledge operations were genetically differentiated and morphologically confirmed. As in Janssen et al.’s (2015) study, they did not find much of a difference between contract areas. Janssen et al. (2015) estimated (based on Chao 1, Chao 2 and Jackknife1) that between 55.6 and 62.7% (Chao 1), 56.1 and 71.1% (Chao 2) and 37.9 and 39% (Jackknife1) of the species from the BGR and IFREMER contract areas have not yet been identified (values reflect numbers for isopods and polychaetes combined). Our results of species estimates of the extended isopod dataset using Chao 1 show that only between 23.6 (UK-1) and 70.0% (BGR) of expected species have been collected so far (Fig. 11). In contrast to the two studies mentioned above, we found a clear difference in diversity between different regions, with the BGR, UK-1 and OMS contract areas showing the lowest diversity, compared to the IFREMER contract area yielding highest species diversity, while the remaining had intermediate diversity levels (Figs. 11 and 12). The low observed diversity of OMS and UK-1 is probably due to the lower sample size overall, and some groups, which are usually very species-rich (e.g. Munnopsidae), were underrepresented in the dataset (Table S2 in the online supplement). Considering contract areas only (BGR, UK-1, OMS, IOM, GSR and IFREMER), estimates range from 309 to 569 ± 72 species (Chao 2: 569 ± 72, Jackknife 1: 394, Jackknife 2: 492, Bootstrap: 309); that is, the estimated percentage of species recovered from the area was between 43.1 (Chao 2) and 79.3% (Bootstrap) of the total observed richness containing 245 MOTUs. In comparison, Brix et al. (2020) estimated (based on Chao 1 and ACE) that between 80.1 and 85.4% of the isopod species have been sampled (Brix et al. 2020, considering BGR, IOM, GSR and IFREMER contract areas combined). Species accumulation curves do not reach an asymptote in any of the studied regions (Fig. 12), and estimates for species richness exceed the observed values in all cases. This, together with the assumption that richness estimators are very sensitive to small numbers and unique occurrences of species (De Smet et al. 2017; Pasotti et al. 2021), indicates that isopod diversity in the CCFZ is still underestimated.
Comparison of Chao 1 richness estimations (± SD) for isopods between different contract areas, APEI#3 and the DISCOL area. Isopod species were defined based on molecular SD methods and partially verified by morphological studies. White dot: number of observed species. Numbers above the bar indicate the estimated proportion of species recovered from the respective area so far
Individual-based rarefaction curves of cumulative number of CCFZ isopod species (MOTUs) per area collected by an epibenthic sledge; red: BGR; orange: DISCOL: black; IOM; green: GSR; turquoise: IFREMER; OMS: grey; UK-1: yellow; blue: APEI#3. For interpretation of the references to colour in this figure legend, the reader is referred to the online version of this article
Wilson (2017) and Washburn et al. (2021b) also found considerable differences in isopod diversity between areas and/or studies. Wilson (2017) examined the diversity of isopods, polychaetes and tanaidaceans in the DOMES, PRA and Echo 1 areas, which show large differences in surface productivity. Notably, his results showed a positive correlation between POC flux and diversity for tanaidaceans and polychaetes, while isopod diversity was negatively correlated with POC input. Despite considering a larger dataset than Wilson’s study from a wider productivity spectrum, Washburn et al. (2021b) could not identify any relationships between isopod diversity and productivity. Indeed, differences in isopod diversity could not be associated with any of the parameters studied, suggesting that other factors, not yet assessed, play a more important role (Washburn et al. 2021b). The inconsistency of results between studies clearly warrants further investigation. However, they all agree on one point; significantly more samples are required to representatively record isopod diversity.
Species geographical distribution, range size and potential drivers
In order to assess the fauna’s potential to recolonise impacted (mining) areas from pristine, protected areas in or bordering the CCFZ (APEIs) or from within the exploration contract areas themselves (e.g. PRZs), knowledge of species range size and its main drivers is required. This relates to the distances that species travel and whether migrations are directional (cf. Taboada et al. 2018). The use of molecular methods in conjunction with the morphological examination of isopod specimens has helped to achieve great advances in delimiting species more robustly and thus better defining their distributional ranges (Janssen et al. 2015, 2019; Brix et al. 2020; Kaiser et al. 2021). Isopod data from the studies presented here indicate that most species appear to have very restricted distributions, limited to a single contract area or even a single sampling station (e.g. Janssen et al. 2015; Brix et al. 2020; Kaiser et al. 2021). Based on molecular-genetic analyses, the assumed widespread distribution of some morpho-species was refuted, revealing that these instead represent morphologically similar or even identical, but genetically different species (Janssen et al. 2015; Kaiser et al. 2021). Notably, some of them were found in close proximity or even sympatrically (i.e. at the same station; Janssen et al. 2015; Kaiser et al. 2021). Furthermore, a high proportion of species were represented by only one or two specimens (termed singletons and doubletons, respectively). For example, Janssen et al. (2015) found that 70% of isopod MOTUs were singletons and are therefore defined as rare until further specimens can be encountered. In comparison, the study by Brix et al. (2020) found that the distribution of 77% of species was restricted to one contract area, and around one-third of these species were represented by only one individual. In our study, which contains significantly more data, 76% of the MOTUs have been collected from a single contract area, and 41.3% are singletons (Fig. 13; Table S2). This presumably high level of locally restricted or “endemic” species results in high turnover rates for isopods in the CCFZ (Janssen et al. 2015; Wilson 2017; Brix et al. 2020; Washburn et al. 2021b). However, it is important to note that the lack of knowledge may lead to the declaration of false endemics and that species may actually have a much wider geographic distribution. Nevertheless, a large proportion of the described species also have small geographical distribution areas and are overall scarce or reveal low numbers of individuals. It is thus likely that most of the hitherto undiscovered species may be rare too (Pimm et al. 2014).
UpSet plot visualising the number of species (resolved by molecular and partially morphological means) shared between different areas. The panel below shows the various possible combinations of the different areas. Species unique to an area are denoted by a dot (vertical bars 1–6), and the corresponding number above the bars indicates the number of unique species found in that area. Species shared between areas are indicated by dots connected by a vertical line, with the number of species co-occurring in each area shown above the vertical bar in the top panel. Created using the web tool: https://gehlenborglab.shinyapps.io/upsetr/ (Lex et al. 2014)
The pattern of small range size and suspected rarity has been contrasted by some isopod species being distributed over large geographical areas within the CCFZ and beyond (Janssen et al. 2015; Brix et al. 2020; Kaiser et al. 2021). For instance, Janssen et al. (2015) found two isopod species to be shared between the BGR and IFREMER contract areas about 1300 km apart. Kaiser et al. (2021) found two species within the family Nannoniscidae to be widespread with haplotypes occurring up to 1400 km apart. Brix et al. (2020) recorded 23% of species occurring in two or more contract areas, consistent with our results. Remarkably, there were seven species that were shared between CCFZ contract areas and the DISCOL area, i.e. about 5000 km apart (Fig. 13). The broadest morphologically and genetically confirmed distribution has been proven for Acanthocope galatheae with known records from the Atlantic and central Pacific oceans (Bober et al. 2018; Brix et al. 2020; S. Bober pers. communication).
It is not clear which mechanisms underlie the observed distribution patterns, but it does not appear to be unique to isopods or the CCFZ. In deep-sea sediments in general, many species seem to be rare, because they were only found in one or two samples or in relatively small amounts (Grassle and Maciolek 1992; Rex and Etter 2010; McClain 2021). On the other hand, a broad distribution has been demonstrated in a range of deep-sea taxa (e.g. McClain & Hardy 2010; Janssen et al. 2015). It is the low sampling effort and the large distances between the samples hitherto taken from the CCFZ that make the evaluation of “true” geographical ranges and thus extent of rarity problematic (Pimm et al. 2014; Pasotti et al. 2021). The widespread distribution of some species of isopods in the CCFZ, on the other hand, appears to be real.
Due to their brooding way of life, the distribution of isopods is thought to be more limited than, for example, the distribution of broadcasting taxa (cf. Janssen et al. 2015; but see Lester et al. 2007). For CCFZ isopods, a strong influence of adult dispersal capacity on species spatial ranges could be ascertained (Brix et al. 2020). For example, the dispersal distances of the less motile Haploniscidae and Macrostylidae were ~ 1400 km compared to > 5000 km for the Munnopsidae, most of which are good swimmer and some of them have excellent swimming abilities (Brix et al. 2020). Several other factors, such as hydrodynamic or topographic conditions and distance between suitable habitats, have been discussed to play important roles in modulating isopod distributions in the CCFZ (Lester et al. 2007; Janssen et al. 2019; Brix et al. 2020; Kaiser et al. 2021). Near-bottom current velocities are fairly low on average but are considered strong enough to allow isopod species to disperse (Janssen et al. 2019). On the other hand, topographical features such as depressions or seamounts can hinder but also facilitate current flow, or the presence of strong oceanic fronts can become a barrier between neighbouring populations and thus impair genetic exchange (Taboada et al. 2018; Janssen et al. 2019). The latter could, inter alia, provide one explanation for population and/or species divergence that was found to occur in isopods on fairly small spatial scales (Janssen et al. 2019; Kaiser et al. 2021). Against the background of the assessment of species ranges, we conclude that species with a good ability to spread have a greater potential to recolonise mined areas, provided that environmental conditions of the affected habitats are restored and that populations of these species thrive in nearby protected areas. However, since the vast majority of isopod species are presumably restricted in their distribution, their diversity could be at risk. To what extent this distribution pattern is real, however, urgently requires further research—with more standardised sampling and analysis needed at multiple spatial scales (i.e. relative to key environmental factors from fine scale [tens of m], local scale [tens of km], to regional scale [hundreds of km], see Simon-Lledó et al. 2020).
Bridging the gaps
The CCFZ has become one of the biologically better-known abyssal areas, although the high proportion of new species (> 90%), but also supra-specific taxa that are still being discovered, emphasises the poor knowledge on diversity and distribution ranges at low taxonomic levels that still exists (e.g. Glover et al. 2018). Despite an ever-increasing number of studies on the CCFZ fauna in the past decade, there are still significant gaps in knowledge and data that do not allow us to make reliable predictions of the potential fauna responses to seabed mining (Hunter et al. 2018; Miller et al. 2018; Amon et al. 2022). After polychaetes, isopods are probably the best-studied macrofaunal taxon in the CCFZ, but taxonomic efforts to describe the isopod fauna in this region have been remarkably poor. It is noteworthy that it was not until 2011 that a first description of an isopod species from the area was published (Malyutina 2011). Ongoing efforts have now led to descriptions of 19 species (Malyutina 2011; Kaiser 2014; Riehl et al. 2014b; Kaiser et al. 2018, 2021; Malyutina et al. 2020; Riehl and De Smet 2020), three genera (Riehl et al. 2014b; Kaiser et al. 2018; Malyutina et al. 2020) and one family (Riehl et al. 2014b) new to science (summarised in Table 2). However, diversity estimates suggest that this is only a minor fraction of the isopod species that actually live there (Janssen et al. 2015; Brix et al. 2020, this study).
Overall, our study shows that knowledge on CCFZ isopods is fragmented and limited to certain contract areas, supra-specific taxa or subsets of fauna. This makes a meaningful comparison between studies and datasets difficult (cf. Washburn et al. 2021b; Fig. 2, Tables 1 and 3). Nevertheless, some trends could be observed, for example that isopod densities appear to be higher in the eastern contract areas than in the more westerly located areas. However, this is not reflected in diversity patterns that show inconsistencies between the studies, and it has not yet been possible to clearly determine, which factors modulate these. There are indications of some widespread species, but the majority of species seems to be locally restricted. A combination of intrinsic (lifestyle) and extrinsic factors and processes, including hydrography, habitat heterogeneity and geographic distance, are likely to play a role in controlling the divergent distribution patterns of isopods. Most importantly, the CCFZ is still severely undersampled and efforts must be made to fill these sampling gaps.
The knowledge gaps identified for the better-known isopods are remarkable in view of the fact that there are a large number of previously neglected groups of organisms, especially among the smaller size fractions that need taxonomic attention (Glover et al. 2016). Furthermore, comparisons with other macrofaunal groups, particularly Polychaeta and Tanaidacea, indicate that one is not an adequate substitute for the other (e.g. Janssen et al. 2015; Wilson 2017; Blazewicz et al. 2019; Washburn et al. 2021b). Therefore, ideally, all of these groups need to be considered to understand how the benthic biodiversity in the CCFZ might be affected by deep-sea mining operations. The intensification of (integrative) taxonomic work, combining morphological, genetic and environmental information, should be promoted to advance knowledge on deep-sea species identities and thus diversity and distributional ranges. Additional samples are just as important as the exchange of samples, experience, know-how and data (accessibility of raw data) between contractors, researchers and research groups to close knowledge gaps and in this way refine our integrated knowledge of distribution patterns in the CCFZ and globally. In addition, sampling design and methods should become standardised in terms of gear type, protocol, mesh size, sample processing and taxonomy in order to enable comparability between studies and to increase the efficiency of concerted scientific efforts and data management. The use of different types of gear (e.g. box corer and epibenthic sledge) is still to be encouraged as these complement each other and will ultimately provide a more comprehensive picture of the CCFZ (isopod) fauna.
This results in the following recommendations for baseline studies:
-
Concomitant use of box corer and epibenthic sledge is recommended as one is not an adequate substitute for the other, but both contribute to a better assessment of macrofaunal diversity patterns.
-
The high spatio-temporal variability observed in our isopod data requires an appropriately adjusted baseline sampling strategy, including sampling across a range of spatial and temporal scales.
-
Efforts should be made to implement standardised sampling and sample processing protocols in order to increase the comparability and informative value of the data.
-
Family-level identification is not sufficient for comparative or impact analyses—a species or MOTU level identification should be achieved. The latter requires taxonomic expertise, which must be guaranteed.
-
Whenever possible, an integrative taxonomic approach should be followed that allows for more robust species identifications. In order to enable both morphological and molecular examination, careful sieving of the benthic material to remove residual sediments and immediate cooling and fixation in high-grade (96%) ethanol are required (Riehl et al. 2014a; Frutos et al. 2022).
-
As elsewhere, most species collected from the abyssal CCFZ have not yet been described, which becomes problematic when comparing samples from different contract areas. To enhance taxonomic inter-calibrations, increased accessibility and query of sample collections and data is a mandatory requirement. Data availability via the ISA DeepData database should therefore be promoted, and DNA barcode reference libraries be expanded.
-
Thorough taxonomic analysis requires time. This is in conflict with ongoing efforts to accelerate biodiversity assessment given the anticipated start of mining activities in the near future. Hence, new methods should be tested, including eDNA, metabarcoding and -omic approaches, alongside more traditional methods to ensure the reliability of identification and delimitation of species (e.g. Lejzerowicz et al. 2021; Frutos et al. 2022; Kürzel et al. 2022).
-
Biodiversity monitoring during exploration and future mining activities in the CCFZ is a long-term activity, spanning several decades of dedicated analysis. Therefore, ideas should be developed to ensure uniform and sustainable taxonomic expertise, for example in the form of workshops offering regular taxonomic inter-calibration exercises or training of the next generation of taxonomists.
-
Most of the available faunal data derive from the central and eastern CCFZ, which leads to a somewhat distorted view of species distributions. Further sampling in the more western contract areas, but also between contract areas and certainly in APEIs, is thus needed to better assess species occurrences and their predicted responses to mining disturbances.
-
Last but not least, assessing possible impacts of mining activities in the CCFZ requires better information about the species living there (Glover et al. 2018). Therefore, in line with the new ISA Sustainable Seabed Knowledge Initiative (https://www.isa.org.jm/sski), species discovery and description should be fostered in conjunction with the development of identification keys that form the basis for effective conservation management.
Abbreviations
- APEI:
-
Area of Particular Environmental Interest
- ABYSSLINE:
-
Abyssal Baseline Programme of UKSRL
- BOLD:
-
Barcode of Life Database
- BGR:
-
Bundesanstalt für Geowissenschaften und Rohstoffe (Federal Institute for Geosciences and Natural Resources)
- CCFZ:
-
Clarion Clipperton Fracture Zone
- COI:
-
Cytochrome c oxidase subunit I
- DISCOL:
-
Disturbance and recolonisation experiment in a manganese nodule area of the deep South Pacific
- DOMES A:
-
Deep Ocean Mining Environmental study site A
- DZMB:
-
German Centre for Marine Biodiversity Research
- Echo 1:
-
Site sampled during ECHO expedition (Spiess et al. 1984) corresponds to DOMES site C (Wilson 2017)
- GSR:
-
Global Sea Mineral Resources NV, DEME group
- IFREMER:
-
L’Institut Français de Recherche pour l’Exploitation de la Mer (French Research Institute for Exploitation of the Sea)
- IOM:
-
Interoceanmetal Joint Organization
- IRZ:
-
Impact Reference Zone
- ISA:
-
International Seabed Authority
- JPIO:
-
Joint Programming Initiative Healthy and Productive Seas and Oceans (JPI Oceans) pilot action “MiningImpact”
- KR:
-
Contract area of the Korean Republic
- MOTU:
-
Molecular taxonomic unit
- NOAA:
-
National Oceanic and Atmospheric Administration
- OMS:
-
Ocean Mineral Singapore Pte. Ltd.
- PA:
-
Prospective Area
- PRA:
-
Preservational Reserve Area Site (termed by NOAA; Wilson 1990)
- PRZ:
-
Preservation Reference Zone
- ROV:
-
Remotely operated vehicle
- UKSRL:
-
UK Seabed Resources Ltd
References
Amon DJ, Ziegler AF, Dahlgren TG, Glover AG, Goineau A et al (2016) Insights into the abundance and diversity of abyssal megafauna in a polymetallic-nodule region in the eastern Clarion-Clipperton Zone. Sci Rep 6:30492. https://doi.org/10.1038/srep30492
Amon DJ, Gollner S, Morato T, Smith CR, Chen C et al (2022) Assessment of scientific gaps related to the effective environmental management of deep-seabed mining. Mar Policy 138:105006. https://doi.org/10.1016/j.marpol.2022.105006
Barnard KH (1920) Contributions to the crustacean fauna of South Africa. No. 6.- Further additions to the list of marine Isopoda. Annls S Afr Mus 17(5):319–438 Plates XV-XVII. Available online at https://www.biodiversitylibrary.org/page/1520873
Błażewicz M, Jóźwiak P, Menot L, Pabis K (2019) High species richness and unique composition of the tanaidacean communities associated with five areas in the Pacific polymetallic nodule fields. Prog Oceanogr 176:102141. https://doi.org/10.1016/j.pocean.2019.102141
Bober S, Brix S, Riehl T, Schwentner M, Brandt A (2018) Does the Mid-Atlantic Ridge affect the distribution of abyssal benthic crustaceans across the Atlantic Ocean? Deep Sea Res II Top Stud Oceanogr 148:91–104. https://doi.org/10.1016/j.dsr2.2018.02.007
Bonifácio P, Martínez Arbizu P, Menot L (2020) Alpha and beta diversity patterns of polychaete assemblages across the nodule province of the eastern Clarion-Clipperton Fracture Zone (equatorial Pacific). Biogeosci 17(4):865–886. https://doi.org/10.5194/bg-17-865-2020
Boyko CB, Bruce NL, Hadfield KA, Merrin KL, Ota Y et al. (Eds) (2008 onwards) World Marine, Freshwater and Terrestrial Isopod Crustaceans database. Asellota. Accessed through: World Register of Marine Species at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=13794. Accessed 25 May 2020
Brandt A (1995) Peracarid fauna (Crustacea, Malacostraca) of the Northeast Water Polynya off Greenland: documenting close benthic-pelagic coupling in the Westwind Trough. Mar Ecol Prog Ser 121:39–51
Brandt A, Brix S, Brökeland W, Choudhury M, Kaiser S et al (2007) Deep-sea isopod biodiversity, abundance, and endemism in the Atlantic sector of the Southern Ocean—results from the ANDEEP I-III expeditions. Deep Sea Res Part II Top Stud Oceanogr 54(16–17):1760–1775. https://doi.org/10.1016/j.dsr2.2007.07.015
Brandt A, Błażewicz-Paszkowycz M, Bamber R, Mühlenhardt-Siegel U, Malyutina M et al (2012) Are there widespread peracarid species in the deep sea (Crustacea: Malacostraca)? Pol Polar Res 33(2):139–162. https://doi.org/10.2478/v10183−012−0012−5
Brandt A, Gutt J, Hildebrandt M, Pawlowski J, Schwendner J et al (2016) Cutting the umbilical: new technological perspectives in benthic deep-sea research. J Mar Sci Eng 4(2):36. https://doi.org/10.3390/jmse4020036
Brenke N (2005) An epibenthic sledge for operations on marine soft bottom and bedrock. Mar Technol Soc J 39(2):10–21. https://doi.org/10.4031/002533205787444015
Brix S, Svavarsson J (2010) Distribution and diversity of desmosomatid and nannoniscid isopods (Crustacea) on the Greenland–Iceland–Faeroe Ridge. Pol Biol 33(4):515–530. https://doi.org/10.1007/s00300-009-0729-8
Brix S, Riehl T, Leese F (2011) First genetic data for species of the genus Haploniscus Richardson, 1908 (Isopoda: Asellota: Haploniscidae) from neighbouring deep-sea basins in the South Atlantic. Zootaxa 2838(1):79–84
Brix S, Leese F, Riehl T, Kihara TC (2015) A new genus and new species of Desmosomatidae Sars, 1897 (Isopoda) from the eastern South Atlantic abyss described by means of integrative taxonomy. Mar Biodiv 45(1):7–61. https://doi.org/10.1007/s12526-014-0218-3
Brix S, Stransky B, Malyutina M, Pabis K, Svavarsson J et al (2018) Distributional patterns of isopods (Crustacea) in Icelandic and adjacent waters. Mar Biodiv 48(2):783–811. https://doi.org/10.1007/s12526-018-0871-z
Brix S, Osborn KJ, Kaiser S, Truskey SB, Schnurr SM et al (2020) Adult life strategy affects distribution patterns in abyssal isopods–implications for conservation in Pacific nodule areas. Biogeosci 17:6163–6184. https://doi.org/10.5194/bg-17-6163-2020
Brix S, Held C, Kaiser S, Jennings RM, Driskell A et al (2021) Evolution and phylogeny of the deep-sea isopod families Desmosomatidae Sars, 1897 and Nannoniscidae Hansen, 1916 (Isopoda: Asellota). Org Div Evol 21:691–717. https://doi.org/10.1007/s13127-021-00509-9
Brökeland W, Raupach MJ (2008) A species complex within the isopod genus Haploniscus (Crustacea: Malacostraca: Peracarida) from the Southern Ocean deep sea: a morphological and molecular approach. Zool J Linn Soc 152(4):655–706. https://doi.org/10.1111/j.1096-3642.2008.00362.x
Brökeland W, Guðmundsson G, Svavarsson J (2010) Diet of four species of deep-sea isopods (Crustacea: Malacostraca: Peracarida) in the South Atlantic and the Southern Ocean. Mar Biol 157(1):177–187. https://doi.org/10.1007/s00227-009-1308-9
Carstens BC, Pelletier TA, Reid NM, Satler JD (2013) How to fail at species delimitation. Mol Ecol 22:4369–4383. https://doi.org/10.1111/mec.12413
Cartes JE, Grémare A, Maynou F, Villora-Moreno S, Dinet A (2002) Bathymetric changes in the distributions of particulate organic matter and associated fauna along a deep-sea transect down the Catalan Sea slope (Northwestern Mediterranean). Prog Oceanog 53(1):29–56. https://doi.org/10.1016/S0079-6611(02)00023-X
Christodoulou M, O’ Hara T, Hugall FA, Khodami S, Rodrigues CF et al (2020) Unexpected high abyssal ophiuroid diversity in polymetallic nodule fields of the northeast Pacific Ocean and implications for conservation. Biogeosci 17:1845–1876. https://doi.org/10.5194/bg-17-1845-2020
Chuar CH, Tong SJW, Chim C, San Wong HP, Tan KS (2020) Abyssal macrofaunal community structure in the polymetallic nodule exploration area at the easternmost region of the Clarion-Clipperton Fracture Zone, Pacific Ocean. Deep Sea Res I Oceanogr Res Pap 161:103284. https://doi.org/10.1016/j.dsr.2020.103284
Clarke KR, Gorley RN (2006) Primer. PRIMER-e, Plymouth, p 866
Cuvelier D, Gollner S, Jones DOB, Kaiser S, Martinez Arbizu P et al (2018) Potential mitigation and restoration actions in ecosystems impacted by seabed mining. Front Mar Sci 5:467. https://doi.org/10.3389/fmars.2018.00467
Dahlgren TG, Wiklund H, Rabone M, Amon DJ, Ikebe C et al (2016) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Cnidaria. Biodivers Data J 4:9277. https://doi.org/10.3897/BDJ.4.e9277
Danovaro R, Gambi C, Dell’Anno A, Corinaldesi C, Fraschetti S et al (2008) Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr Biol 18:1–8. https://doi.org/10.1016/j.cub.2007.11.056
De Smet B, Pape E, Riehl T, Bonifácio P, Colson L et al (2017) The community structure of deep-sea macrofauna associated with polymetallic nodules in the eastern part of the Clarion-Clipperton Fracture Zone. Front Mar Sci 4:103. https://doi.org/10.3389/fmars.2017.00103
Dellicour S, Flot JF (2018) The hitchhiker’s guide to single-locus species delimitation. Mol Ecol Res 18(6):1234–1246. https://doi.org/10.1111/1755-0998.12908
Dietz L, Arango CP, Dömel JS, Halanych KM, Harder AM et al (2015) Regional differentiation and extensive hybridization between mitochondrial clades of the Southern Ocean giant sea spider Colossendeis megalonyx. R Soc Open Sci 2(7):140424. https://doi.org/10.1098/rsos.140424
Durden JM, Murphy K, Jaeckel A, Van Dover CL, Christiansen S et al (2017) A procedural framework for robust environmental management of deep-sea mining projects using a conceptual model. Mar Pol 84:193–201. https://doi.org/10.1016/j.marpol.2017.07.002
Elsner NO, Malyutina MV, Golovan OA, Brenke N, Riehl T et al (2015) Deep down: isopod biodiversity of the Kuril-Kamchatka abyssal area including a comparison with data of previous expeditions of the RV Vityaz. Deep Sea Res II Top Stud Oceanogr 111:210–219. https://doi.org/10.1016/j.dsr2.2014.08.007
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Fritz JS (2016) Commentary: Threatened by mining, polymetallic nodules are required to preserve abyssal epifauna. Front Mar Sci 3:190. https://doi.org/10.3389/fmars.2016.00190
Frutos I, Kaiser S, Pułaski L, Studzian M, Błażewicz M (2022) Challenges and advances in the taxonomy of deep-sea Peracarida: from traditional to modern methods. Front Mar Sci 9:799191. https://doi.org/10.3389/fmars.2022.799191
Fujisawa T, Barraclough TG (2013) Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: a revised method and evaluation on simulated data sets. Syst Biol 62:707–724. https://doi.org/10.1093/sysbio/syt033
Glover AG, Smith CR, Paterson GLJ, Wilson GDF, Hawkins L et al (2002) Polychaete species diversity in the central Pacific abyss: local and regional patterns, and relationships with productivity. Mar Ecol Progr Ser 240:157–170. https://doi.org/10.3354/meps240157
Glover A, Dahlgren T, Taboada S, Paterson G, Wiklund H et al (2016) The London workshop on the biogeography and connectivity of the Clarion-Clipperton Zone. Research Ideas and Outcomes 2:e10528. https://doi.org/10.3897/rio.2.e10528
Glover AG, Wiklund H, Chen C, Dahlgren TG (2018) Point of view: managing a sustainable deep-sea ‘blue economy’ requires knowledge of what actually lives there. Elife 7:e41319. https://doi.org/10.7554/eLife.41319
Glover AG, Higgs N, Horton T (2021) World Register of Deep-Sea species (WoRDSS). Accessed at http://www.marinespecies.org/deepsea on 2021–09–15. https://doi.org/10.14284/352
Gollner S, Govenar B, Martinez Arbizu P, Mills S, Le Bris N et al (2015) Differences in recovery between deep-sea hydrothermal vent and vent-proximate communities after a volcanic eruption. Deep Sea Res II Top Stud Oceanogr 106:167–182. https://doi.org/10.1016/j.dsr.2015.10.008
Gollner S, Kaiser S, Menzel L, Jones DOB, Brown A et al (2017) Resilience of benthic deep-sea fauna to mining activities. Mar Environ Res 129:76–101. https://doi.org/10.1016/j.marenvres.2017.04.010
Golovan OA, Błażewicz M, Brandt A, Jażdżewska AM, Jóźwiak P et al (2019) Diversity and distribution of peracarid crustaceans (Malacostraca) from the abyss adjacent to the Kuril-Kamchatka Trench. Mar Biodiv 49(3):1343–1360. https://doi.org/10.1007/s12526-018-0908-3
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4(4):379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Grassle JF, Maciolek NJ (1992) Deep-sea species richness: regional and local diversity estimates from quantitative bottom samples. Am Nat 139(2):313–341
Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Hessler RR, Jumars PA (1974) Abyssal community analysis from replicate cores in the central North Pacific. Deep Sea Res Oceanogr Abstr 21(3):185–209
Hessler RR, Wilson GDF, Thistle D (1979) The deep-sea isopods: a biogeographic and phylogenetic overview. Sarsia 64(1–2):67–75
Hessler RR, Wilson GDF (1983) The origin and biogeography of malacostracan crustaceans in the deep sea. In: Sims RW, Price JH, Whalley PES (eds) Evolution, time and space: the emergence of the biosphere. Academic Press, London, pp 227–254
Hunter J, Singh P, Aguon J (2018) Broadening common heritage: addressing gaps in the deep-sea mining regulatory regime. Harv Environ Law Rev 16:1–12
International Seabed Authority (ISA) (2020) Recommendations for the guidance of contractors for the assessment of the possible environmental impacts arising from exploration for marine minerals in the Area. https://www.isa.org.jm/wp-content/uploads/2022/06/26ltc-6-rev1-en_0.pdf
International Seabed Authority (ISA) (2021) Decision of the Council of the International Seabed Authority relating to the review of the environmental management plan for the Clarion-Clipperton Zone. ISBA/26/C/58. International Seabed Authority, Kingston, Jamaica. https://www.isa.org.jm/wp-content/uploads/2022/06/ISBA_26_C_58_E.pdf
International Seabed Authority (ISA) (2000) Regulations on prospecting and exploration for polymetallic nodules in the area, ISBA/6/A/18 (13 July 2000), amended by ISBA/19/A/9; ISBA/19/A/12 (25 July 2013) and ISBA/20/A/9 (24 July 2014) (Nodules Exploration Regulations), 2014.
International Seabed Authority (ISA) (2011) Environmental management plan for the Clarion Clipperton Zone. ISBA/17/LTC/7, International Seabed Authority, Kingston, Jamaica
Janssen A, Kaiser S, Meissner K, Brenke N, Menot L et al (2015) A reverse taxonomic approach to assess macrofaunal distribution patterns in abyssal Pacific polymetallic nodule fields. Plos one 10(2):e0117790. https://doi.org/10.1371/journal.pone.0117790
Janssen A, Stuckas H, Vink A, Martinez Arbizu P (2019) Biogeography and population structure of predominant macrofaunal taxa (Annelida and Isopoda) in abyssal polymetallic nodule fields: implications for conservation and management. Mar Biodiv 49(6):2641–2658. https://doi.org/10.1007/s12526-019-00997-1
Janssen A, Albers L, Ostmann A, Raschka U (2013) Benthic biodiversity. MANGAN 2013 cruise report. BGR, Hannover, p 13
Janssen A, Kaiser S, Albers L, Mohrbeck I, Raschka U (2014) Benthic biodiversity. MANGAN 2014 cruise report. BGR, Hannover, p 12
Jennings RM, Golovan O, Brix S (2020) Integrative species delimitation of desmosomatid and nannoniscid isopods from the Kuril-Kamchatka trench, with description of a hadal species. Progr Oceanogr 182:102236. https://doi.org/10.1016/j.pocean.2019.102236
Jones DOB, Kaiser S, Sweetman AK, Smith CR, Menot L et al (2017) Biological responses to disturbance from simulated deep-sea polymetallic nodule mining. PLoS ONE 12(2):e0171750. https://doi.org/10.1371/journal.pone.0171750
Jones DOB, Durden JM, Murphy K, Gjerde KM, Gebicka A et al (2019) Existing environmental management approaches relevant to deep-sea mining. Mar Pol 103:172–181. https://doi.org/10.1016/j.marpol.2019.01.006
Jones DOB, Ardron JA, Colaço A, Durden JM (2020) Environmental considerations for impact and preservation reference zones for deep-sea polymetallic nodule mining. Mar Pol 118:10331. https://doi.org/10.1016/j.marpol.2018.10.025
Jóźwiak P, Pabis K, Brandt A, Błażewicz M (2020) Epibenthic sled versus giant box corer–comparison of sampling gears for tanaidacean species richness assessment in the abyssal benthic ecosystem. Progr Oceanogr 181:102255. https://doi.org/10.1016/j.pocean.2019.102255
Kaiser S (2014) New species of Hebefustis Siebenaller & Hessler 1977 (Isopoda, Asellota, Nannoniscidae) from the Clarion Clipperton Fracture Zone (equatorial NE Pacific). Zootaxa 3784(2):101–119. https://doi.org/10.11646/zootaxa.3784.2.1
Kaiser S, Brenke N (2016) Epibenthic sledges. Biological sampling in the deep sea. Wiley Blackwell, West Sussex, pp 184–206
Kaiser S, Barnes DKA, Brandt A (2007) Slope and deep-sea abundance across scales: Southern Ocean isopods show how complex the deep sea can be. Deep Sea Res II Top Stud Oceanogr 54(16–17):1776–1789. https://doi.org/10.1016/j.dsr2.2007.07.006
Kaiser S, Smith CR, Martinez Arbizu P (2017) Editorial: Biodiversity of the Clarion Clipperton Fracture Zone. Mar Biodiv 47(2):259–264. https://doi.org/10.1007/s12526-017-0733-0
Kaiser S, Brix S, Kihara TC, Janssen A, Jennings RM (2018) Integrative species delimitation in the deep-sea genus Thaumastosoma Hessler, 1970 (Isopoda, Asellota, Nannoniscidae) reveals a new genus and species from the Atlantic and central Pacific abyss. Deep Sea Res II Top Stud Oceanogr 148:151–179. https://doi.org/10.1016/j.dsr2.2017.05.006
Kaiser S, Menot L, Błażewicz-Paszkowycz M, Bonifacio P, Neal L et al. (2015) Macrofauna. In: Martínez Arbizu P, Haeckel M (eds): RV SONNE Fahrtbericht/Cruise Report SO239: EcoResponse Assessing the ecology, connectivity and resilience of polymetallic nodule field systems, Balboa (Panama)–Manzanillo (Mexico,) 11.03.-30.04. 2015: 76–103. https://doi.org/10.3289/GEOMAR_REP_NS_25_2015.
Kaiser S, Kihara TC, Brix S, Mohrbeck I, Janssen A et al. (2021) Species boundaries and phylogeographic patterns in new species of Nannoniscus (Janiroidea: Nannoniscidae) from the equatorial Pacific nodule province inferred from mtDNA and morphology. Zool J Linn Soc 193(3): 1020–1071. https://doi.org/10.1093/zoolinnean/zlaa174
Kapli P, Lutteropp S, Zhang J, Kobert K, Pavlidis P et al (2017) Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 33:1630–1638. https://doi.org/10.1093/bioinformatics/btx025
Knox MA, Hogg ID, Pilditch CA, Lörz AN, Nodder SD (2012) Abundance and diversity of epibenthic amphipods (Crustacea) from contrasting bathyal habitats. Deep Sea Res I Oceanogr Res Pap 62:1–9. https://doi.org/10.1016/j.dsr.2011.12.011
Kuhnz LA, Ruhl HA, Huffard CL, Smith KL Jr (2014) Rapid changes and long-term cycles in the benthic megafaunal community observed over 24 years in the abyssal northeast Pacific. Progr Oceanogr 124:1–11. https://doi.org/10.1016/j.pocean.2014.04.007
Kürzel K, Kaiser S, Lörz AN, Rossel S, Paulus E et al. (2022) Correct species identification and its implications for conservation using Haploniscidae (Crustacea, Isopoda) in Icelandic waters as a proxy. Front Mar Sci 8:795196. https://doi.org/10.3389/fmars.2021.795196
Kussakin OG (1973) Peculiarities of the geographical and vertical distribution of marine isopods and the problem of deep-sea fauna origin. Mar Biol 23(1):19–34
Lampitt RS, Billett DSM, Rice AL (1986) Biomass of the invertebrate megabenthos from 500 to 4100 m in the northeast Atlantic Ocean. Mar Biol 93(1):69–81
Lejzerowicz F, Gooday AJ, Barrenechea Angeles I, Cordier T, Morard R et al (2021) Eukaryotic biodiversity and spatial patterns in the Clarion-Clipperton zone and other abyssal regions: insights from sediment DNA and RNA metabarcoding. Front Mar Sci 8:536. https://doi.org/10.3389/fmars.2021.671033
Lester SE, Ruttenberg BI, Gaines SD, Kinlan BP (2007) The relationship between dispersal ability and geographic range size. Ecol Lett 10(8):745–758. https://doi.org/10.1111/j.1461-0248.2007.01070.x
Levin LA, Etter RJ, Rex MA, Gooday AJ, Smith CR et al (2001) Environmental influences on regional deep-sea species diversity. Annu Rev Ecol Systemat 32:51–93
Levin LA, Mengerink K, Gjerde KM, Rowden AA, Van Dover CL et al (2016) Defining “serious harm” to the marine environment in the context of deep-seabed mining. Mar Pol 74:245–259. https://doi.org/10.1016/j.marpol.2016.09.032
Lex A, Gehlenborg N, Strobel H, Vuillemo R, Pfister H (2014) UpSet: visualization of intersecting sets. IEEE Trans Visual Comput Graphics 20(12):1983–1992. https://doi.org/10.1109/TVCG.2014.2346248
Lins L, Brandt A (2020) Comparability between box-corer and epibenthic-sledge data on higher taxon level: a case study based on deep-sea samples from the NW Pacific. Prog Oceanogr 182:102273. https://doi.org/10.1016/j.pocean.2020.102273
Lins LS, Ho SY, Wilson GDF, Lo N (2012) Evidence for Permo-Triassic colonization of the deep sea by isopods. Biol Lett 8(6):979–982. https://doi.org/10.1098/rsbl.2012.0774
Lins L, Zeppilli D, Menot L, Michel LN, Bonifácio P et al. (2021) Toward a reliable assessment of potential ecological impacts of deep-sea polymetallic nodule mining on abyssal infauna. Limnol Oceanogr Meth 19:626–650. https://doi.org/10.1002/lom3.10448
Lodge M, Johnson D, Le Gurun G, Wengler M, Weaver P et al (2014) Seabed mining: International Seabed Authority environmental management plan for the Clarion-Clipperton Zone. A Partnership Approach Mar Pol 49:66–72. https://doi.org/10.1016/j.marpol.2014.04.006
Lörz AN, Kaiser S, Bowden D (2013) Macrofaunal crustaceans in the benthic boundary layer from the shelf break to abyssal depths in the Ross Sea (Antarctica). Pol Biol 36(3):445–451. https://doi.org/10.1007/s00300-012-1269-1
Magoga G, Fontaneto D, Montagna M (2021) Factors affecting the efficiency of molecular species delimitation in a species-rich insect family. Mol Ecol Res 21(5):1475–1489. https://doi.org/10.1111/1755-0998.13352
Magurran AE (2004) Measuring biological diversity. Blackwell Science, Oxford
Malyutina MV (2011) Description of two new species of munnopsid isopods (Crustacea: Isopoda: Asellota) from manganese nodules area of the Clarion-Clipperton Fracture Zone. Pacific Ocean. Zootaxa 2783(1):1–20. https://doi.org/10.11646/zootaxa.2783.1.1
Malyutina MV, Brandt A (2006) A revaluation of the Eurycopinae (Crustacea, Isopoda, Munnopsidae) with a description of Dubinectes gen. nov. from the southern Atlantic deep sea. Zootaxa 1272(1):1–44
Malyutina MV, Brandt A (2018) First records of deep-sea Munnopsidae (Isopoda: Asellota) from the Kuril Basin of the Sea of Okhotsk, with description of Gurjanopsis kurilensis sp. nov. Deep Sea Res II Topical Stud Oceanogr 154:275–291. https://doi.org/10.1016/j.dsr2.2017.12.006
Malyutina MV, Brandt A (2020) Munnopsidae (Crustacea, Isopoda, Asellota) from the Kuril-Kamchatka Trench with a regional and inter-ocean comparison of their biogeographic and richness patterns. Progr Oceanogr 183:102289. https://doi.org/10.1016/j.pocean.2020.102289
Malyutina M, Kihara TC, Brix S (2020) A new genus of Munnopsidae Lilljeborg, 1864 (Crustacea, Isopoda), with descriptions of two abyssal new species from the Clarion Clipperton Fracture Zone, north-eastern tropical Pacific. Mar Biodiv 50:1–31. https://doi.org/10.1007/s12526-020-01061-z
Martínez Arbizu P, Haeckel M (2015) RV SONNE Fahrtbericht/Cruise Report SO239: EcoResponse Assessing the ecology, connectivity and resilience of polymetallic nodule field systems, Balboa (Panama)–Manzanillo (Mexico,) 11.03.-30.04. 2015. GEOMAR Report, N. Ser. 025. GEOMAR Helmholtz-Zentrum für Ozeanforschung, Kiel, Germany, p 204. https://doi.org/10.3289/GEOMAR_REP_NS_25_2015
McClain CR (2021) The commonness of rarity in a deep-sea taxon. Oikos 130(6):863–878. https://doi.org/10.1111/oik.07602
McClain CR, Hardy SM (2010) The dynamics of biogeographic ranges in the deep sea. Proc R Soc b: Biol Sci 277(1700):3533–3546. https://doi.org/10.1098/rspb.2010.1057
McQuaid KA, Attrill MJ, Clark MR, Cobley A, Glover AG et al (2020) Using habitat classification to assess representativity of a protected area network in a large, data-poor area targeted for deep-sea mining. Front Mar Sci 7:1066. https://doi.org/10.3389/fmars.2020.558860
Menot L, Galéron J, Rühlemann C, Albers L, Briand P et al (2013) BIONOD cruise report, vol 2. Ifremer, French claim area, p 81
Menzies RJ, George RY (1972) Isopod Crustacea of the Peru-Chile Trench. Anton Bruun Report 9:1–124
Meyer-Löbbecke A, Brandt A, Brix S (2014) Diversity and abundance of deep-sea Isopoda along the Southern Polar Front: results from the SYSTCO I and II expeditions. Deep Sea Res II Top Stud Oceanogr 108:76–84. https://doi.org/10.1016/j.dsr2.2014.06.006
Miller KA, Thompson KF, Johnston P, Santillo D (2018) An overview of seabed mining including the current state of development, environmental impacts, and knowledge gaps. Front Mar Sci 4:418. https://doi.org/10.3389/fmars.2017.00418
Niner HJ, Ardron JA, Escobar EG, Gianni M, Jaeckel A et al (2018) Deep-sea mining with no net loss of biodiversity—an impossible aim. Front Mar Sci 5:53. https://doi.org/10.3389/fmars.2018.00053
Osborn KJ (2009) Relationships within the Munnopsidae (Crustacea, Isopoda, Asellota) based on three genes. Zool Scr 38(6):617–635. https://doi.org/10.1111/j.1463-6409.2009.00394.x
Pape E, De Smet B, Bogaert K, Vanreusel A (2016) Biological and environmental report on the 2014 and 2015 expeditions in the GSR license area. Marine Biology Research Group, Ghent University, Ghent, Belgium, p 147
Pape E, De Smet B, Gheerardyn H, Bezerra TN, Vanreusel A (2017) Report on the continued analyses of the biological and environmental samples collected during the 2015 expedition in the GSR exploration area. Ghent University, Marine Biology Research Group, p 132
Pape E, De Smet B, Gheerardyn G, Lefaible N, Mevenkamp L et al (2018) Report on the continued analyses of the biological and environmental samples collected during the 2015 and 2017 expeditions in the GSR license area. Ghent University, Marine Biology Research Group, p 120
Pasotti F, Mevenkamp L, Pape E, Błażewicz M, Bonifácio P et al (2021) A local scale analysis of manganese nodules influence on the Clarion-Clipperton Fracture Zone macrobenthos. Deep Sea Res I Oceanogr Res Pap 168:103449. https://doi.org/10.1016/j.dsr.2020.103449
Paulus E, Brix S, Siebert A, Martinez Arbizu P, Rossel S et al (2022) Recent speciation and hybridization in Icelandic deep-sea isopods: an integrative approach using genomics and proteomics. Mol Ecol 31(1):313–330. https://doi.org/10.1111/mec.16234
Peukert A, Schoening T, Alevizos E, Köser K, Kwasnitschka T et al (2018) Understanding Mn-nodule distribution and evaluation of related deep-sea mining impacts using AUV-based hydroacoustic and optical data. Biogeosciences 15(8):2525–2549. https://doi.org/10.5194/bg-15-2525-2018
Pfenninger M, Schwenk K (2007) Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evol Biol 7(1):1–6. https://doi.org/10.1186/1471-2148-7-121
Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL et al. (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Science 344(6187). https://doi.org/10.1126/science.1246752
Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP et al (2006) Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst Biol 55:595–609. https://doi.org/10.1080/10635150600852011
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256. https://doi.org/10.1093/molbev/msn083
Puillandre N, Brouillet S, Achaz G (2021) ASAP: assemble species by automatic partitioning. Mol Ecol Res 21:609–620. https://doi.org/10.1111/1755-0998.13281
Rabone M, Horton T, Jones DO, Simon-Lledó E, Glover AG (2022) A review of the International Seabed Authority database DeepData: challenges and opportunities in the UN Ocean Decade. bioRxiv. https://doi.org/10.1101/2022.10.14.512288
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogeneomics using Tracer 1.7. Syst Biol 67:901–904. https://doi.org/10.1093/sysbio/syy032
Ratnasingham S, Hebert PDN (2013) A DNA-based registry for all animal species: the barcode index number (BIN) system. PLoS ONE 8:e66213. https://doi.org/10.1371/journal.pone.0066213
Raupach MJ, Malyutina M, Brandt A, Wägele JW (2007) Molecular data reveal a highly diverse species flock within the munnopsoid deep-sea isopod Betamorpha fusiformis (Barnard, 1920) (Crustacea: Isopoda: Asellota) in the Southern Ocean. Deep Sea Res II Top Stud Oceanogr 54(16–17):1820–1830. https://doi.org/10.1016/j.dsr2.2007.07.009
Raupach MJ, Mayer C, Malyutina M, Wägele JW (2009) Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data. Philos Trans R Soc Lond B Biol Sci 276(1658):799–808. https://doi.org/10.1098/rspb.2008.1063
Rex MA, Etter RJ (2010) Deep-sea biodiversity: pattern and scale. Harvard University Press
Rex MA, Stuart CT, Hessler RR, Allen JA, Sanders HL et al (1993) Global-scale latitudinal patterns of species diversity in the deep-sea benthos. Nature 365(6447):636–639. https://doi.org/10.1038/365636a0
Rex MA, Etter RJ, Morris JS, Crouse J, McClain CR et al (2006) Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar Ecol Progr Ser 317:1–8. https://doi.org/10.3354/meps317001
Ribardière A, Daguin-Thiébaut C, Houbin C, Coudret J, Broudin C et al (2017) Geographically distinct patterns of reproductive isolation and hybridization in two sympatric species of the Jaera albifrons complex (marine isopods). Ecol Evol 7(14):5352–5365. https://doi.org/10.1002/ece3.3106
Riehl T, De Smet B (2020) Macrostylis metallicola spec nov—an isopod with geographically clustered genetic variability from a polymetallic-nodule area in the Clarion-Clipperton Fracture Zone. Peer J 8:e862. https://doi.org/10.7717/peerj.8621
Riehl T, Kaiser S (2012) Conquered from the deep sea? A new deep-sea isopod species from the Antarctic shelf shows pattern of recent colonization. PLoS One 7(11):e49354. https://doi.org/10.1371/journal.pone.0049354
Riehl T, Kühn MA (2020) Uniting what belongs together—reevaluation of the isopod species Macrostylis grandis and M ovata using ontogenetic, morphological and genetic evidence. Prog Oceanogr 181:102238. https://doi.org/10.1016/j.pocean.2019.102238
Riehl T, Wilson GDF, Hessler RR (2012) New Macrostylidae Hansen, 1916 (Crustacea: Isopoda) from the Gay Head-Bermuda transect with special consideration of sexual dimorphism. Zootaxa 3277(1):1–26
Riehl T, Brenke N, Brix S, Driskell A, Kaiser S et al (2014a) Field and laboratory methods for DNA studies on deep-sea isopod crustaceans. Pol Polar Res 35(2):203–224. https://doi.org/10.2478/popore−2014−0018
Riehl T, Wilson GDF, Malyutina MV (2014b) Urstylidae—a new family of abyssal isopods (Crustacea: Asellota) and its phylogenetic implications. Zool J Linn Soc 170(2):245–296. https://doi.org/10.1111/zoj.12104
Riehl T, Bober S, Voltski I, Malyutina MV, Brandt A (2018a) Caught in the act: an abyssal isopod collected while feeding on Komokiaceae. Mar Biodiv 48:157–158. https://doi.org/10.1007/s12526-016-0606-y
Riehl T, Lins L, Brandt A (2018b) The effects of depth, distance, and the Mid-Atlantic Ridge on genetic differentiation of abyssal and hadal isopods (Macrostylidae). Deep-Sea Res Pt II Topical Stud Oceanogr 148:74–90. https://doi.org/10.1016/j.dsr2.2017.10.005
Rühlemann C, Baumann L, Blöthe M, Bruns A, Eisenhauer A et al (2010) Cruise report SO-205 MANGAN, microbiology, paleoceanography and biodiversity in the manganese nodule belt of the equatorial NE Pacific. BGR, Hannover, p 113
Rühlemann C, Albers L, Briand P, Brulport JP, Cosson R et al (2012) BIONOD Cruise report. BGR, Hannover, p 299
Rühlemann C, Bruns A, Edullantes C, Ercan T, Gatzemeier N et al (2019) MANGAN 2018 cruise report: geology, biodiversity and environment of the German license area for the exploration of polymetallic nodules in the equatorial NE Pacific. Bundesanstalt für Geowissenschaften und Rohstoffe, Hannover, p 324
Schlacher TA, Baco AR, Rowden AA, O’Hara TD, Clark MR et al (2014) Seamount benthos in a cobalt-rich crust region of the central Pacific: conservation challenges for future seabed mining. Div Distrib 20(5):491–502. https://doi.org/10.1111/ddi.12142
Schnurr S, Osborn KJ, Malyutina M, Jennings R, Brix S et al (2018) Hidden diversity in two species complexes of munnopsid isopods (Crustacea) at the transition between the northernmost North Atlantic and the Nordic Seas. Mar Biodiv 48(2):813–843. https://doi.org/10.1007/s12526-018-0877-6
Simon-Lledó E, Pomee C, Ahokava A, Drazen JC, Leitner AB et al (2020) Multi-scale variations in invertebrate and fish megafauna in the mid-eastern Clarion Clipperton Zone. Progr Oceanogr 187:102405. https://doi.org/10.1016/j.pocean.2020.102405
Singh R, Miljutin DM, Vanreusel A, Radziejewska T, Miljutina MM et al (2016) Nematode communities inhabiting the soft deep-sea sediment in polymetallic nodule fields: do they differ from those in the nodule-free abyssal areas? Mar Biol Res 12(4):345–359. https://doi.org/10.1080/17451000.2016.1148822
Singh R, Sautya S, Ingole BS (2019) The community structure of the deep-sea nematode community associated with polymetallic nodules in the Central Indian Ocean Basin. Deep-Sea Res Pt II Topical Stud Oceanogr 161:16–28. https://doi.org/10.1016/j.dsr2.2018.07.009
Singh PA (2021) The two-year deadline to complete the International Seabed Authority’s Mining Code: key outstanding matters that still need to be resolved. Mar Pol 134. https://doi.org/10.1016/j.marpol.2021.104804
Smith CR, Gaines S, Friedlander A, Morgan C, Thurnherr A, Mincks S et al (2008) Rationale and recommendations for the establishment of preservation reference areas for nodule mining in the Clarion-Clipperton Zone. Technical document no. ISBA/14/LTC/2. Fourteenth session. Legal and Technical Commission, International Seabed Authority, Kingston, Jamaica. https://www.isa.org.jm/files/documents/EN/14Sess/LTC/ISBA-14LTC-2.pdf
Song H, Buhay JE, Whiting MF, Crandall KA (2008) Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc Natl Acad Sci USA 105:13486–13491. https://doi.org/10.1073/pnas.0803076105
Spiess FN, Hessler R, Wilson G, Weydert M, Rude P (1984) ECHO I Cruise Report vol 84-3. Scripps Institution of Oceanography, La Jolla, California. https://doi.org/10.13140/RG.2.1.4131.0242
Stuart CT, Rex MA, Etter RJ (2003) Large-scale spatial and temporal patterns of deep-sea benthic species diversity. In: Tyler PA (ed) Ecosystems of the deep oceans. Ecosystems of the World, vol 28. Elsevier, Amsterdam, pp 295–312
Stucky BJ (2012) SeqTrace: a graphical tool for rapidly processing DNA sequencing chromatograms. J Biomol Techn 23(3):90. https://doi.org/10.7171/jbt.12-2303-004
Svavarsson J, Stromberg JO, Brattegard T (1993) The deep-sea asellote (Isopoda, Crustacea) fauna of the Northern Seas: species composition, distributional patterns and origin. J Biogeogr 537–555. https://doi.org/10.2307/2845725
Taboada S, Riesgo A, Wiklund H, Paterson GL, Koutsouveli V et al (2018) Implications of population connectivity studies for the design of marine protected areas in the deep sea: an example of a demosponge from the Clarion-Clipperton Zone. Mol Ecol 27(23):4657–4679. https://doi.org/10.1111/mec.14888
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Tang CQ, Humphreys AM, Fontaneto D, Barraclough TG, Paradis E (2014) Effects of phylogenetic reconstruction method on the robustness of species delimitation using single-locus data. Methods Ecol Evol 5:1086–1094. https://doi.org/10.1111/2041-210X.12246
Taylor J, Krumpen T, Soltwedel T, Gutt J, Bergmann M (2017) Dynamic benthic megafaunal communities: assessing temporal variations in structure, composition and diversity at the Arctic deep-sea observatory HAUSGARTEN between 2004 and 2015. Deep-Sea Res Pt I Oceanogr Res Papers 122:81–94. https://doi.org/10.1016/j.dsr.2017.02.008
Thistle D, Wilson GDF (1987) A hydrodynamically modified, abyssal isopod fauna. Deep Sea Res Part A Oceanogr Res Papers 34(1):73–87
Thistle D, Wilson GDF (1996) Is the HEBBLE isopod fauna hydrodynamically modified? A second test. Deep Sea Res Pt I Oceanogr Res Pap 43(4):545–554
Thurber AR, Sweetman AK, Narayanaswamy BE, Jones DOB, Ingels J et al (2014) Ecosystem function and services provided by the deep sea. Biogeosci 11(14):3941–3963. https://doi.org/10.5194/bg-11-3941-2014
Uhlenkott K, Mohrbeck I, Albers L, Iwan F, Weerts F et al (2016) Biodiversity of benthic fauna within the polymetallic nodule belt, NE Pacific (Clarion Clipperton Fracture Zone). MANGAN 2016, BGR-Abschlussbericht, Hanover, p 70
Uhlenkott K, Janssen A, Raschka U, Egho G, Menke S et al (2017) Biodiversity of benthic fauna within the polymetallic nodule belt, NE Pacific (Clarion Clipperton Fracture Zone). MANGAN 2017, BGR-Abschlussbericht, Hanover, p 115
Uhlenkott K, Khodami S, Menke S, Gatzemeier N, Mercado Salas N et al (2018) Biodiversity of benthic fauna within the polymetallic nodule belt, NE Pacific (Clarion Clipperton Fracture Zone). MANGAN 2018, BGR-Abschlussbericht, Hanover, p 108
Van Dover CL, Ardron JA, Escobar E, Gianni M, Gjerde KM et al (2017) Biodiversity loss from deep-sea mining. Nature Geosci 10(7):464–465. https://doi.org/10.1038/ngeo2983
Vanreusel A, Hilario A, Ribeiro PA, Menot L, Martinez Arbizu P (2016) Threatened by mining, polymetallic nodules are required to preserve abyssal epifauna. Sci Rep 6(1):1–6. https://doi.org/10.1038/srep26808
Veillette J, Sarrazin J, Gooday AJ, Galéron J, Caprais JC et al (2007) Ferromanganese nodule fauna in the Tropical North Pacific Ocean: species richness, faunal cover and spatial distribution. Deep Sea Res Pt I Oceanogr Res Pap 54(11):1912–1935. https://doi.org/10.1016/j.dsr.2007.06.011
Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10(4):506–513
Washburn TW, Jones DOB, Wei CL, Smith CR (2021a) Environmental heterogeneity throughout the Clarion-Clipperton Zone and the potential representativity of the APEI network. Front Mar Sci 8:319. https://doi.org/10.3389/fmars.2021.661685
Washburn TW, Menot L, Bonifácio P, Pape E, Błażewicz M et al (2021b) Patterns of macrofaunal biodiversity across the Clarion-Clipperton Zone: an area targeted for seabed mining. Front Mar Sci 8:250. https://doi.org/10.3389/fmars.2021.626571
Wedding LM, Friedlander AM, Kittinger JN, Watling L, Gaines SD et al (2013) From principles to practice: a spatial approach to systematic conservation planning in the deep sea. Proc Royal Soc B 280(1773):20131684. https://doi.org/10.1098/rspb.2013.1684
Wedding LM, Reiter SM, Smith CR, Gjerde KM, Kittinger JN et al (2015) Managing mining of the deep seabed. Sci 349(6244):144–145. https://doi.org/10.1126/science.aac6647
Wilson GDF (1990) Biological evaluation of a preservational reserve area “BEPRA I.” Cruise report and interim report on laboratory analysis, Scripps Institution of Oceanography, p 34
Wilson GDF (1991) Functional morphology and evolution of isopod genitalia. In: Crustacean sexual biology. Columbia University Press, New York/Oxford, pp 228–245
Wilson GDF (1992) Biological evaluation of a preservational reference area—faunal data and comparative analysis. Australian Museum, Sydney
Wilson GDF (1998) Historical influences on deep-sea isopod diversity in the Atlantic Ocean. Deep Sea Res Pt II Top Stud Oceanogr 45(1–3):279–301. https://doi.org/10.1016/S0967-0645(97)00046-5
Wilson GDF (1999) Some of the deep-sea fauna is ancient. Crustaceana 72(8):1019–1030
Wilson GDF (2017) Macrofauna abundance, species diversity and turnover at three sites in the Clipperton-Clarion Fracture Zone. Mar Biodiv 47(2):323–347. https://doi.org/10.1007/s12526-016-0609-8
Wilson GDF (2008) A review of taxonomic concepts in the Nannoniscidae (Isopoda, Asellota), with a key to the genera and a description of Nannoniscus oblongus Sars. Zootaxa 1680(1):1–24
WoRMS Editorial Board (2021) World Register of Marine Species. Available from https://www.marinespecies.org at VLIZ. Accessed 2021–09–24. https://doi.org/10.14284/170
Würzberg L, Peters J, Brandt A (2011) Fatty acid patterns of Southern Ocean shelf and deep sea peracarid crustaceans and a possible food source, foraminiferans. Deep Sea Res Pt II Top Stud Oceanogr 58(19–20):2027–2035. https://doi.org/10.1016/j.dsr2.2011.05.013
Yoder M, De Ley IT, King IW, Mundo-Ocampo M, Mann J et al (2006) DESS: a versatile solution for preserving morphology and extractable DNA of nematodes. Nematol 8(3):367–376. https://doi.org/10.1163/156854106778493448
Yu OH, Lee HG, Kim D, Wi JH, Kim KH et al (2018) Characterization of deep-sea macrofauna in the Korean exploration claim area in the Clarion-Clipperton Fracture Zone, northeastern Pacific Ocean. Ocean Sci J 53(2):301–314. https://doi.org/10.1007/s12601-018-0029-8
Acknowledgements
We would like to thank the masters, crew and participants of RV Kilo Moana, RV Sonne, RV L’Atalante, RV Thomas G. Thompson, MV Mt. Mitchell and MV Topaz Captain during various sampling campaigns to the CCFZ for their logistical support and help while collecting the isopod material. Over the course of different projects, Uwe Raschka (Senckenberg), Karen Osborn (Smithsonian, Washington D.C.) and Lisa Mevenkamp (UGhent) helped generate the genetic dataset, while Nils Brenke, Saskia Brix, Torben Riehl (Senckenberg), Lisa Mevenkamp and Bart De Smet (UGhent) partly conducted the morphological examinations. Muriel Rabone (NHM London) is thanked for making the (cleaned) ISA DeepData dataset for isopods available, while Paulo Bonifacio is thanked for providing a high-resolution map of the sampling area. We notably thank Carsten Rühlemann and Thomas Kuhn (BGR), Lenaick Menot (IFREMER), Matthias Haeckel (GEOMAR), Craig Smith (SOEST, University of Hawaii), Ann Vanreusel and Ellen Pape (UGhent) for their efforts in coordinating the MANGAN, BIONOD, JPIO MiningImpact, ABYSSLINE and GSR projects, respectively. We furthermore thank the BGR, GSR and UKSRL for sharing their data. This is publication number 92 that uses data from the Senckenberg am Meer Metabarcoding and Molecular Laboratory. We thank two anonymous reviewers for their constructive comments on an earlier version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding has been furthermore received from the German Ministry of Education and Science (BMBF) as a contribution to the European projects JPI Oceans “MiningImpact” (under contract 03F0707E) and “MiningImpact 2” (under contract 03F0812E). The BIONOD expedition was funded in equal shares by the BGR and IFREMER, and the MANGAN expeditions including faunal analysis of sampled material were funded solely by the BGR. We also are grateful for the support of this work through a commercial arrangement with UKSRL. The expeditions and lab analyses of GSRNOD15A and GSRNOD17 samples were funded by GSR as part of the environmental baseline survey carried out by GSR and as part of a service agreement between GSR and Ghent University. SK acknowledges a grant from the Narodowa Agencja Wymiany Akademickiej (Poland) under the ULAM program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with unregulated invertebrate species.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the “Acknowledgements”, if applicable.
Data availability
All faunistic data generated or analysed during this study are included in this published article, whereas COI sequences are deposited in GenBank (see electronic supplementary Table S2). The physical specimens that were not used for taxonomic descriptions are located either at the DZMB in Wilhelmshaven (contact: Pedro Martinez Arbizu) or the Marine Biology Research Group of Ghent University (contact: Ellen Pape). Type material of the described species is either stored at the Senckenberg Museum, Frankfurt (SMF), or the Crustacean Collection of the Museum of Nature (Leibniz Institute for the Analysis of Biodiversity Change, LIB) (formerly Center of Natural History, CeNak) in Hamburg.
Author contribution
SK performed the literature search and wrote the manuscript with the help of all co-authors; MC and SK conducted the data analysis; MC was responsible for coordinating and managing the sequence data; AJ, IM, SMS, TCK and FP performed the molecular laboratory work; AV and PMA conceived and designed the research. All authors read and approved the manuscript.
Additional information
Communicated by T. Horton
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the Topical Collection Biodiversity in Abyssal Polymetallic Nodule Areas
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaiser, S., Christodoulou, M., Janssen, A. et al. Diversity, distribution and composition of abyssal benthic Isopoda in a region proposed for deep-seafloor mining of polymetallic nodules: a synthesis. Mar. Biodivers. 53, 30 (2023). https://doi.org/10.1007/s12526-023-01335-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-023-01335-2