Abstract
Background
Alanine aminotransferase (ALT) is widely used to screen patients with hepatic diseases. However, the current reference ranges (< 50 U/L) were developed by laboratories and have not been validated in populations with a large number of healthy individuals.
Methods
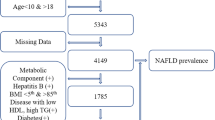
This study collected venous blood and anthropometric data from a total of 13,287 healthy children aged 3 months to 18 years who underwent routine physical examinations in the Department of Pediatric Healthcare. We applied the least mean square algorithm to establish age- and sex-related reference percentiles of serum levels of transaminases. For validation, we recruited 4276 children and adolescents with obesity/overweight who underwent evaluation and metabolic tests in the hospital. Using receiver operating characteristic curves, we determined age- and sex-specific upper limit percentiles of liver enzymes for fatty liver diseases.
Results
This study revealed a significant correlation between serum transaminase levels and age and sex (P < 0.01). These transaminase levels exhibited age- and sex-specific patterns. Among individuals in the non-alcoholic fatty liver disease (NAFLD) cohort, elevated ALT levels displayed a positive association with clinical markers of disease severity, including homeostatic model assessment of insulin resistance, waist–hip ratio, and serum uric acid levels (P < 0.01). According to the receiver operating characteristic curves, ALT levels at the 92.58th percentile for boys and the 92.07th percentile for girls yielded the highest accuracy and specificity.
Conclusions
This study provides age- and sex-specific reference ranges for ALT, aspartate aminotransferase, and γ-glutamyltransferase in Chinese children and adolescents, making it the largest population study to date. Furthermore, the study establishes a precise upper limit for ALT levels, facilitating their use in NAFLD screening.
Video Abstract




Similar content being viewed by others
Data availability
The data sets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol. 2019;16:517–30.
Hesham A-Kader H. Nonalcoholic fatty liver disease in children living in the obeseogenic society. World J Pediatr. 2009;5:245–54.
Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38.
Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35.
Yasuda Y, Miyake N, Matsuoka H, Sugihara S. Adiponectin, ALT and family history as critical markers for the development of type 2 diabetes in obese Japanese children. Endocrinol Diabetes Metab. 2021;4:e00178.
Kang Y, Park S, Kim S, Koh H. Normal serum alanine aminotransferase and non-alcoholic fatty liver disease among Korean adolescents: a cross-sectional study using data from KNHANES 2010–2015. BMC Pediatr. 2018;18:215.
Lu Y, Wang Q, Yu L, Yin X, Yang H, Xu X, et al. Revision of serum ALT upper limits of normal facilitates assessment of mild liver injury in obese children with non-alcoholic fatty liver disease. J Clin Lab Anal. 2020;34:e23285.
Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138:64.e1–2.
Stirnadel-Farrant HA, Galwey N, Bains C, Yancey C, Hunt CM. Children’s liver chemistries vary with age and gender and require customized pediatric reference ranges. Regul Toxicol Pharmacol. 2015;73:349–55.
Estey MP, Cohen AH, Colantonio DA, Chan MK, Marvasti TB, Randell E, et al. CLSI-based transference of the CALIPER database of pediatric reference intervals from Abbott to Beckman, Ortho, Roche and Siemens Clinical Chemistry Assays: direct validation using reference samples from the CALIPER cohort. Clin Biochem. 2013;46:1197–219.
Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–7.
Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape. J R Stat Soc Ser C Appl Stat. 2005;54:507–54.
R Core Team. R: A language and environment for statistical computing R foundation for statistical computing. Vienna, Austria. 2013.
Jin B, Wu Z, Wang S, Yu Z, Ullah R, Liang X, et al. Gender differences in non-alcoholic fatty liver disease in obese children and adolescents: a large cross-sectional study. Hepatol Int. 2023. https://doi.org/10.1007/s12072-023-10596-9.
Kohse KP, Thamm M. KiGGS-the German survey on children’s health as data base for reference intervals. Clin Biochem. 2011;44:479.
Pan XB, Lu YQ, Lin SZ, Ye J, Wu N, Lou YY, et al. An assessment of upper limits of normal for ALT and the impact on evaluating natural course of chronic hepatitis B virus infection in Chinese children. Am J Gastroenterol. 2018;113:1660–8.
Bussler S, Vogel M, Pietzner D, Harms K, Buzek T, Penke M, et al. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase): effects of age, sex, body mass index, and pubertal stage. Hepatology. 2018;68:1319–30.
Ratra S, Dhingra K, Sharma S, Gupta GK, Nijhawan S. Levels of aminotransferases among schoolchildren in Jaipur Rajasthan. Indian Pediatr. 2021;58:683–4.
Li X, Wang D, Yang C, Zhou Q, Zhuoga SL, Wang LQ, et al. Establishment of age- and gender-specific pediatric reference intervals for liver function tests in healthy Han children. World J Pediatr. 2018;14:151–9.
Hilsted L, Rustad P, Aksglæde L, Sørensen K, Juul A. Recommended nordic paediatric reference intervals for 21 common biochemical properties. Scand J Clin Lab Invest. 2013;73:1–9.
Hoq M, Matthews S, Karlaftis V, Burgess J, Cowley J, Donath S, et al. Reference values for 30 common biochemistry analytes across 5 different analyzers in neonates and children 30 days to 18 years of age. Clin Chem. 2019;65:1317–26.
Qin ZW, Ren QN, Zhang HX, Liu YR, Huang K, Wu W, et al. Development and validation of a novel non-invasive test for diagnosing nonalcoholic fatty liver disease in Chinese children. World J Pediatr. 2023. https://doi.org/10.1007/s12519-023-00704-y.
Hong Y, Ullah R, Wang JB, Fu JF. Trends of obesity and overweight among children and adolescents in China. World J Pediatr. 2023;19:1115–26.
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20.
Funding
This study was supported by grants from the National Key Research and Development Program of China (Nos. 2021YFC2701901 and 2016YFC1305301), National Natural Science Foundation of China (Nos. 82370863 and 81570759), and Zhejiang Provincial Key Disciplines of Medicine (Innovation Discipline, 11-CX24).
Author information
Authors and Affiliations
Contributions
WZY contributed to investigation, conceptualization, resources, data curation, methodology, formal analysis, validation, writing of the original draft, reviewing and editing. CSW and OLJ contributed to investigation, conceptualization, methodology, formal analysis, validation, writing of the original draft, reviewing and editing. XXQ, NY and SQ contributed to investigation, conceptualization, resources, and data curation. CJN and JBH contributed to writing of the original draft, reviewing and editing. UR contributed to investigation, conceptualization, writing of the original draft, reviewing and editing. ZXL, HK, WW, DGP, LZM, SY, SJ contributed to resources and data curation. FJF and WW contributed to investigation and conceptualization, and was responsible for funding acquisition and supervision. All authors critically reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Zhejiang University (2021-IRB-301). Informed consent to participate in the study has been obtained from participants (or their parent or legal guardian in the case of children under 16).
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. Authors Jun-Fen Fu and Qiang Shu are member of the Editorial Board and Chief Editor for World Journal of Pediatrics, respectively. This paper was handled by the other Editor and has undergone rigorous peer-review process. Authors Jun-Fen Fu and Qiang Shu were not involved in the journal’s review of, or decisions related to, this manuscript. The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, ZY., Chi, SW., Ouyang, LJ. et al. Continuous age- and sex-specific reference ranges of liver enzymes in Chinese children and application in pediatric non-alcoholic fatty liver disease. World J Pediatr (2024). https://doi.org/10.1007/s12519-023-00789-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12519-023-00789-5