Abstract
Pollution from shipwrecking is growing into a major occupational and environmental health concern worldwide, especially in the lower-middle-income countries where workers and residents may be exposed to potentially high levels of dispersed metal/loids due to decades of improper waste disposal. This study is the first to report the urinary biomarkers estimating the exposure of metal/loids and associated significant determinants in residents working and/or living at and near a major open beaching shipwrecking yard in Bangladesh. The concentrations of 17 elements were measured in urine and drinking water samples (125 each) using a validated ICP-MS method. Hydration variations of urine were best adjusted by specific gravity. Significantly higher (p < 0.001) urinary metal/loids load including cadmium and molybdenum in the occupational (70–80% of shipwrecking workers) and environmental exposure groups (72–75% of the local population) compared to the control site and international reference values suggest a significant health threat. Gender and age effects on the variation of urinary metal/loid concentrations were insignificant. However, fertilizer/pesticide users had higher nickel concentrations. Quantile regression analyses suggested the impacted locations with shipwrecking activities are significant predictors of exposure. The participants from the shipwrecking yards and the adjoining east, north, and south sites experienced significant exposure to metal/loids, which may be largely governed by wind-associated dispersion of contaminants. Water metal/loid concentrations were lower than the guideline values set by WHO and Bangladesh except for manganese in 63% of water samples. However, the elevated urinary concentrations of cadmium and thallium were significantly correlated to their concentration in water. This biomonitoring approach could be used as a background study to facilitate a more detailed exposure and health risk assessment to inform a management strategy for residents exposed to contaminated environmental matrices and dietary sources.
Similar content being viewed by others
Introduction
Shipwrecking, also known as ship recycling (ILO 2004), dismantles end-of-life (EOL) ships after a mean economic lifespan of 20–30 years. It is becoming a major occupational and environmental health problem globally (ILO 2015) due to the release of large amounts of carcinogens and toxic substances, including polychlorinated biphenyls (PCBs), polyvinyl chlorides (PVCs), polycyclic aromatic hydrocarbons (PAHs), tri-butyl-tin (TBT), and metal/loids (Barua et al. 2018; Gunbeyaz et al. 2019). Elements such as cadmium (Cd), mercury (Hg), molybdenum (Mo), etc., are used in different parts of a ship (Barua et al. 2018; Hossain et al. 2016; Kakar et al. 2021).
Occupational workers are likely exposed to mixed metal/loids in the workplace of shipwrecking yards (SWYs) through inhalation (airborne particulates, fume in air) and dermal contact (soil/dust, aerosol, direct contact with wastes during ship dismantling), incidental ingestion of contaminated soil particles, consumption of drinking water, and food from the impacted area. Other residents from the surrounding yards can be exposed to the metal/loids through exposure to groundwater, rice, vegetables, seafood, air, and soil/dust.
Globally the steady flow of EOL ships requires competent shipwrecking yards (SWYs) to recover the valuable materials and return these substances to the economic material flow (Sujauddin et al. 2017). However, the majority of developing countries (like Bangladesh, India, Pakistan, and China) recycled 90–95% of EOL ships using an open-beaching method due to cost-effective operations, cheaper labor costs, and very relaxed environmental safety and occupational health regulations (Barua et al. 2018; Gunbeyaz et al. 2019; Platform 2019). Open-beaching shipwrecking is a faster dismantling process but can be detrimental to humans and environmental health due to the uncontrolled release of hazardous wastes (Alam et al. 2019; Deshpande et al. 2012). The SWYs in Bangladesh are concentrated along the coastline of Sitakund (sub-district), and Chittagong (Chattogram), wherein a significant amount (around 31%) of EOL ships being wrecked on the open beach, with very little or no containment and occupational safety measures for the workers are often neglected (Gunbeyaz et al. 2019). There were 419 EOL ships dismantled in Bangladesh from 2018 to 2019 alone (Barua et al. 2018; Platform 2019). It is one of the booming industrial sectors in Bangladesh with enormous employment opportunities. It has provided up to 60% of the total steel usage in the country (Alam et al. 2019; Barua et al. 2018; Hossain et al. 2016) over the last five decades.
The major concern is that a significant amount of uncontrolled waste materials may disperse into the soil, water, sediment, and air in the surrounding environment of the SWYs (Barua et al. 2018; Sarraf et al. 2010; Sujauddin et al. 2015) and can significantly affect the surrounding environment, the local communities, fisheries, agriculture, flora, and fauna (ILO 2015). Notably, the metal/loids from the wastes may accumulate in soil or find their way into the seawater, groundwater, sediment, and air. Finally, the metals may enter the human body through the food chain, leading to potential health risks to the occupational workers and local populations due to their environmental persistence, toxicity, and bioaccumulation (Hasan et al. 2020; Kakar et al. 2021; Kumar et al. 2019).
Although the amounts of mixed metals released from the shipwrecking activities are unknown, quantifying contamination levels in its surrounding environment may provide valuable information on the effect of past and current activities. A few previous studies in Bangladesh and India have suggested potential health risks (such as respiratory, skin, and kidney disease) due to harmful metals from SWYs through pathways including soil, sediment, seawater, groundwater, air, and food (Alam et al. 2019; Basha et al. 2007; Hasan et al. 2020; Hossain et al. 2016). However, urinary biomonitoring of occupational and non-occupational exposure to metal/loids in the shipwrecking yard in Bangladesh, even in the world, is lacking. To the best of our knowledge, this is the first biomonitoring study of its kind. The objectives of the study were to (1) determine the level of urinary metal/loids in occupational workers and also in non-occupational people living adjoining mega-scale SWYs in Chittagong (Chattogram), Bangladesh, (2) investigate the influence of the demographic characteristic (age, sex, occupation, smoking status, pesticide/fertilizer use, etc.) and climatic factor (wind speed and direction) on the accumulated metal/loid concentrations, and (3) identify the significant contributing factors to the metal and metalloid exposure. The present biomonitoring study may serve as an informative database for using urine as a biomarker for future biomonitoring programs.
Materials and Methods
Study Area
The shipwrecking yards (SWYs) in Bangladesh are mainly concentrated in Sitakund (sub-district), an administrative unit located in the north of Chittagong (Chattogram) district (city) on the coast of the Bay of Bengal in Bangladesh with a total area of 4,893.97 km2. The current study area lies between the coordinates of latitudes 22° 22′ N to 22° 41′ N and longitudes 91° 35′ E to 91° 46′ E. The shipwrecking operations started in 1972. The second-largest SWY in the world is located in this area after the largest one in Alang-Sosiya, India. The SWYs provided 51% of the country’s requirement for scrap iron in steel industries and 37% of the total need for finished steel products (Hossain & Islam 2006; Ministry of Industries 2011; Sujauddin et al. 2017). It is estimated that there are around 145 SWYs (84 functional) in Sitakund, which spans over 16 km, along with 25,000 employees directly and 200,000 people indirectly involved in SW-related activities (Barua et al. 2018; Islam et al. 2013).
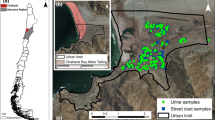
Five exposure sites were selected at and near the open beaching ship wrecking yards (within a 40 km radius circle around the SWYs) in Chittagong (Chattogram) based on their significance, nature of the matrices, the intensity of agricultural farm, and locally grown foods and wind aloft flow pattern. The study sites are (1) SWYs (spans over 16 km, Faujdarhat to Kumira, Sitakund) and their nearby surrounding sites, namely (2) East (E), (3) West (W), (4) North (N), and (5) South (S), respectively, which covered ~ 15 km (Basbaria to Fatehabad, Hathazari), ~ 18 km (Santoshpur to Sarikait, Sandwip), ~ 20 km (Barabkunda, Sitakund to Hatikandi, Mirsharai), and ~ 17 km (Salimpur, Sitakund to Uttar Kattali, Chittagong City), respectively (Fig. 1). The SWY is in the center of the study area and is considered an occupational exposure group. The surrounding East, West, North, and South sites are regarded as an environmental (or non-occupational) exposure group. The west (W) site is an Island called Sandwip, a sub-district, a non-industrial and remote area of 762.4 km2, which is around 25 km away from the SWYs. Approximately 1 million (1,052,576) people live in the whole study area (Bangladesh Bureau of Statistics 2014). This area’s people mainly depend on agriculture and fisheries; some are involved in industrial manufacturing (steel re-rolling and food processing), shipwrecking (SW) industries, and related businesses for their livelihood. The west site’s (Site-W) people depend on agriculture and fisheries.
Google Earth map, a indicates Bangladesh, b exposure sites in Chittagong and control site (CS) in Barguna district, c five exposure sites sampling locations marked by the shipwrecking yard, SWY (red-colored), and the surrounding east (E, purple colored), north (N, yellow colored), south (S, blue colored), and west (W, orange colored) sites
To compare the study results with reference values, Barguna Sadar (sub-district) (lies between 22.16° to 22.02° N and 90.10° to 90.02° E), Barguna, in the southern part of Bangladesh, with an area of 454.39 km2 and a population of about 219,729, was selected as a reference control site. It is a semi-rural, non-industrial area with insignificant arsenic contamination in Bangladesh (Chakraborti et al. 2010). The control site is approximately 250 km away from the exposure sites. This area is surrounded by an estuary system where the freshwater of the Payra and Bishkhali rivers flows upstream to the Bay of Bengal. It is considered a suitable control site not affected by anthropogenic industrial activities except for agricultural activities. The people of this area mainly depend on agriculture and fisheries.
The climate of the study area is characterized by tropical hot and humid summers from April to June of 25–32 °C, and wet and warm monsoon continues from July to October of 26–30 °C with a predominating south-north directed average wind speed of 16 km/h. Also, the study area is characterized by mild winter from November to March of 16–30 °C with a prevalent switching over the wind of north–south directed, average speed 9 km/h (Mark Jacobson et al. 2018; Windrose 2020). A wind rose is shown in Fig. S1.
Demographic Characteristics and Sampling Procedure
The study area participants were 60% male, 40% female, aged 20 to 65 years (median age 39.6 years) and resided for at least three years. The participants (n = 100) were randomly selected and collected urine samples representing the occupationally exposed SWY’s group and their adjacent four sites of the environmentally exposed groups in the study area (20 samples from each site). In addition, 25 participants (male 56%, female 44%, aged 22 to 68 years, median age 39.5) were randomly selected from the control group. The participants’ demographic characteristics were also surveyed (Table 1). Detailed demographic information (age, gender, residence time, alcohol consumption and smoking habits, fertilizer/pesticide use, betel nut use, occupation) was obtained using a questionnaire (Sanchez et al. 2018). For the association of urinary metal/loids levels with drinking water, 125 drinking water samples were also collected from participants’ home private tube wells or the public water distribution systems at the five exposure sites (20 samples from each exposure site) and control site (25 samples). The sample size was at least 20 samples (p < 0.05) per sampling site determined by G*Power 3.1 using independent t tests where effect sizes were calculated from a mean and standard deviation of two groups/sites from related literature (Aguilera et al. 2010; Asante et al. 2012; Berglund et al. 2011; Khlifi et al. 2014; Sanchez et al. 2018; Tanvir et al. 2021). The study protocols were approved by the Ethics Committee, Directorate of Research and Extension, Chittagong Veterinary and Animal Sciences University, Chittagong (Chattogram), Bangladesh (Memo no.-CVASU/Dir(R&E) EC/2020/191/3). All participants provided oral-informed consent.
The first-void morning urine samples were collected, representing the elemental exposure from the last work shift and possibly the last 24 h. The urine samples were collected from the participants in sterile polyethylene containers pre-treated with nitric acid (10% v/v) and rinsed three times with Milli-Q water (18.25 MΩ.cm). The collected samples were filtered using MF-Millipore™ filter units (mixed cellulose esters, pore size: 0.45 μm, Merck, Germany). Aliquots (10 mL) of the samples were acidified to a concentration of 1% HNO3 (v/v) with concentrated HNO3. The remaining unacidified collected samples were stored at − 20 °C in the Biomaterials Research Lab ACCE, University of Chittagong, Bangladesh. The samples were then packed with the Brazilian cooler thermo-gel, GELO-X, and shipped to Australia. Samples were stored at -20 ˚C until analysis at QAEHS laboratory, The University of Queensland, Australia. Drinking water samples (n = 125) were also collected from the study and control areas, filtered and preserved in 1% HNO3 (v/v), stored, and transported to QAEHS in the same manner.
Quantification of Metal/Loid Concentrations in Urine and Water Samples
Seventeen elemental concentrations (Cd, Hg, V, Cr, Mn, Fe, Co, Ni, Sr, Mo, Sn, Sb, Cs, Ba, Tl, U, and As) in the urine were measured by inductively coupled plasma-mass spectrometry (Agilent 7900 ICP-MS, Agilent Technologies, Japan), equipped with a fourth-generation Octapole Reaction System (ORS4) operated in helium collision mode (Supplementary Information SI 1). This study only used total arsenic (As) concentrations for urine dilution correction. According to the principle and modification of published procedures, the ICP-MS method for urinary metals was developed and validated by comparing matrix-matched and heat digestion methods (Freire et al. 2018; Heitland & Köster 2004; Tanvir, et al. 2020).
Chemical reagents, sample preparation for matrix-matched and heat digestion methods, and preparation of external calibration standards are presented in the Supplementary Information SI 2, SI 3, and SI 4, respectively. The certified reference material (CRM), Seronorm™ Trace Elements Urine L-1, and spiking solutions (pooled urine with standards) were included for the method validation. The matrix-matched method provided comparatively better analytical quality, faster and simplified urinary metal/loids analysis with excellent linearity (r2 ≥ 0.9999), sensitivity (lower LOD—limit of detection), recoveries (between 95 and 109%), and precision (lowest coefficient of variation (CV %) for intra-day repeatability, < 6.7%, and inter-day reproducibility, < 9.8%) for the certified reference material (CRM) and spike solutions compared to that of heat digestion. More information on quality assurance data is presented in SI 5. Most of the measured elements were well above their calculated LOD, except V (15%), Cr (39%), Sn (45%), Sb (11%), Ba (27%), Hg (27%), and U (12%) (Table S10).
The specific gravity (SG) and creatinine (CR) methods were comparatively assessed in hydration correcting concentrations of total urinary arsenic (As) in study participants exposed to different levels of As from private drinking water supplies. The specific gravity was measured by a temperature-corrected, handheld UG-α digital refractometer (Atago, Japan) (Middleton et al. 2019). Creatinine (CR) concentration was quantified following kinetic colorimetric assay of Jaffe method using the OLYMPUS creatinine assay kit on a Beckman Coulter AU480 chemistry analyzer (Beckman Coulter Inc. Brea California, USA) (Middleton et al. 2019) (SI 6). The specific gravity adjustment showed the most appropriate rather than the widely used creatinine judging by the scatter plot analyses (SI 6 and Fig. S2), which agrees with previous studies (Concha et al. 2010; Middleton et al. 2019).
The concentrations of 17 metal/loid (including Pb, Se, Cu, and Zn) in drinking water samples were also measured by ICP-MS followed by the U.S. environmental protection agency (U.S. EPA) guidelines (Creed et al. 1994) and a published procedure (Ciminelli et al. 2017). The certified reference material (CRM), TM 24.3 (Lot 0510, Environment Canada), trace elements, and spiking solutions (water samples with standards) were included for the method validation. The method showed better analytical quality and simplified metal/loids analysis in water with excellent linearity (r2 ≥ 0.9999), sensitivity (lower LOD—limit of detection), recoveries (between 91 and 113%), and precision (lowest coefficient of variation (CV %) for intra-day repeatability, < 4.5%, and inter-day reproducibility, < 8.9%) for the certified reference material (CRM) and spike solutions, respectively. More information on metal/loid in drinking water analysis is shown in SI 7. The linearity, sensitivities, accuracy, and precision of the method are presented in Tables S6 and S7.
Data Analysis
The study area map was prepared using Arc GIS (version Pro 2.7, Esri). Statistical analyses and data visualization were performed using R software (R studio, R version 4.0.2), GraphPad Prism (version 9.0.0, GraphPad Inc), SPSS (IBM SPSS Statistics 27), and MS Office Excel. The distributions of urinary and drinking water metal concentrations were right-skewed, and thus non-parametric tests were performed. Geometric mean and percentiles were calculated for metal concentrations in the samples. The elemental concentrations below LOD were denoted by LOD/2 for statistical calculation (Paschal et al. 1998; Tanvir et al. 2020). For selecting the urine dilution adjustment method and an association of metals between urine and water, the urine and drinking water metal/loids concentrations were log-transformed to achieve normality, and the scatter plots were constructed (Middleton et al. 2019). The variation of urinary metal and drinking water elements concentrations between or among independent groups were tested using Mann–Whitney’s U (GraphPad Prism) or Kruskal Wallis’s test (SPSS). The box whisker charts of the urinary metal concentrations were plotted to identify the study sites’ variation trends (using R). Quantile regression analysis was used to identify significant contributing factors to the urinary metal levels (SPSS). Spearman’s correlation test was used for univariate associations (SPSS). The statistical significance is defined in this study as a p value between < 0.001 and < 0.05.
Power analysis and bootstrap estimates of the sample data were also assessed to overcome small sample issues in this study (SPSS). Although the sample size was relatively small, the study afforded meaningful observation of power (86–95%) for the metal/loids of interest with an α = 0.05 using 20–25 samples at each site to indicate significant differences as calculated using G*power 3.1. Also, the bootstrap bias-corrected and accelerated (BCa) method produces confidence intervals (CI) with bootstrap resampling (n = 1000) that have good coverage probabilities under a small size sample distribution. It is a robust method that provides approximately true estimates representing the population parameters (Burch 2012; Nicolae et al. 2013). In this study, we used 1000 replication (resampling) of the sample of 20–25 at each site from the study empirical sample to calculate the bootstrap median concentration of metal/loids with bootstrap BCa 95% CI. The median estimates and 95% CI calculated with the traditional approach were compared with the bootstrapped estimates and bootstrap BCa 95% CI. We did not find any significant difference between the traditional and bootstrap estimates (Table S14). Thus, traditional estimates are reported in this study. However, bootstrap estimates are also reported in the supplementary Table S14. Therefore, both estimates provided valid observation with what seems to be a relatively small sample size which was necessarily consistent with the sample size required for representativeness.
Results and Discussion2
Demography of the Participants
The participants’ demography and clinical parameters are presented in Table 1. The variations in elemental concentrations in urine by demographic characteristics are also provided in Table S8. The age and gender (except SWY site, primarily male workers employed with at least three years of job experience) of the residents in the exposure site and control site were no significant differences. Around 70% of the participants lived in the exposure sites permanently (by birth), except 30% in the SWY, whereas the rest were for 3–10 years. However, there was no significant difference in the residence times between the exposure (except SWY site, a considerable portion of residents for 3–10 years) site and control site (p > 0.05). There were 39% current smokers with male predominated, 20% fertilizer/pesticide users, and 28% betel nut consumers. Alcohol consumption of the participants was infrequent in the study area. The average creatinine concentration was 0.96 (range, 0.17–3.1) g/L. Six participants had urine creatinine concentration below 0.3 g/L, and one had more than 3.0 g/L, and they were excluded for minimizing the over dilution correction (Berglund et al. 2011; Protano et al. 2016).
There were no significant differences in the elemental concentrations between current smokers and non-smokers, especially non-significant effects of smoking on the urinary cadmium. The gender and age of the participants had no significant effect on most of the urinary metal concentrations. Fertilizer/pesticide users had higher Ni (p < 0.05) levels compared to non-users (SI 8 and Table S8).
Betel nut, a mild stimulant drug, users had no elevated values compared to non-users. After adjusting this covariate (betel nut consumption) in the regression model, there was no significant relationship between betel nut users and metal/loid exposure (Tables S8 and 2). Betel nut chewing with betel-leaf has been practiced for hundreds of years; it is a very common social habit in South Asian countries, including Bangladesh and India (Al-Rmalli et al. 2011b). It has been reported that betel nut use could be associated with human exposure to manganese, arsenic, lead, and cadmium (Al-Rmalli et al. 2011a, b; Sanchez et al. 2018). Therefore, it is considered a subgroup for the elevated contribution to the exposure of the metal/loids.
Urinary creatinine is a marker of muscle mass, strongly associated with body mass index (BMI) (Bansal et al. 2011; Baxmann et al. 2008). The creatinine concentration has also been influenced by demographic characteristics (Barr et al. 2005), protein, and meat intake (Baxmann et al. 2008). It is an indicator of nutritional and bodily function status (Tanvir et al. 2021). It is also considered as a covariate for the alternative of BMI.
Urinary Metal/Loid Levels in the Occupationally and /or Environmentally Exposed Groups
The variations of urinary metal/loid concentrations among the occupational (SWYs site) and environmental exposure groups (Site-E, Site-W, Site-N, and Site-S) and control group (CS) (or references values in the USA, UK, Germany, French, Belgium, and Korean adult populations) are shown in Fig. 2 and Tables S9. Also, a comparison of urinary metal/loid concentrations between the cumulated sites of the exposure groups (5 sites altogether) and the control group are shown in S10. Most of the urinary metal/loid levels in our control group agreed with the reference values in the different countries (Table S9). Furthermore, the control group’s urinary metal/loid concentrations agreed with the element values of non-industrial, rural areas of Bangladeshi adults (Berglund et al. 2011). Therefore, the control site urinary metal/loid values were adopted and used as a reference control group to compare with occupational and environmental exposure groups.
Variations of urinary metal/loid concentrations among the occupational (SWY site) and environmental (non-occupational) exposure groups (Site-E, Site-W, Site-N, and Site-S) and control group. Where single (*), double (**), and triple asterisk (***) represent at p < 0.05, p < 0.01, and p < 0.001, respectively. All elemental concentrations were standardized by specific gravity
Significantly higher concentrations (p < 0.001) of urinary V were found in the participants from the occupational group (Site SWY), environmental groups of Site-E (p < 0.01) and Site-N (p < 0.001) compared to the control site (CS) (or, other countries’ reference values) (Fig. 2, Table S9). Elevated concentrations (p < 0.0001) of V were also found in cumulated sites of the exposure groups compared to CS (Table S10). However, higher urinary excretion of V may be associated with elevated levels in seafood (Ebrahimi Yazdanabad et al. 2014) that may be due to contamination of crude oil waste from shipwrecking activities. The urinary V levels of most of the people (85%) were within the normal human urine value (0.5 µg/L) (ATSDR 2012b). In addition, V is used as an alloy substance to improve steel properties, and shipwrecking activities may influence the release of V in the environment.
Levels of Co of participants were markedly higher in SWY (p < 0.01), Site-N (p < 0.001), Site-S (p < 0.001), and Site-W (p < 0.05) compared to CS and other reference values (Fig. 2, Table S9). Elevated concentrations (p < 0.0001) of Co were also found in cumulated sites of the exposure groups compared to CS (Table S10). Urinary Co is associated with skin exposure and may be considered a biomarker of exposure (Kettelarij et al. 2018). Concentrations of urinary Mo were remarkably higher in SWY (p < 0.05), Site-E (p < 0.001), Site-W (p < 0.01), and cumulated sites of the study area (p < 0.0001) compared to that of CS, and other reference values. It is important to note that the Mo levels in 80% of the residents were higher than the normal value (32 µg/L) (ATSDR 2011). It is distributed in the environment through industrial and agricultural contamination (Smedley & Kinniburgh 2017). Acute higher exposure to Mo has been related to diarrhea, anemia, and gout, where occupational exposure has been linked to weakness, anorexia, liver dysfunction, joint pain, osteoporosis, and tremor (WHO 2004). However, diets are the primary sources of these metals, and extreme urinary excretion of these elements could be associated with elevated abundance in the environment and foods due to improper waste management of shipwrecking activities. Although small amounts of these dietary elements of Co and Mo are essential for life, extreme exposure to these elements could be toxic and may lead to death (Briffa et al. 2020).
Significantly higher levels of urinary Cd were found in the participants from SWY (p < 0.05), Site-E (p < 0.01), Site-S (p < 0.05), and Site-W (p < 0.01) of exposure groups compared to that of the control group, and reference values (Fig. 2, Table S9). Significantly higher concentrations of Cd (p < 0.0001) were also observed in cumulated sites of the exposure groups compared to CS (Table S10). Urinary Cd levels in most participants were higher than the normal populations’ (≥ 6 years of age) levels (0.185 µg/L) (ATSDR 2011). There was a non-significant effect of smoking on the urinary Cd among people in this present study (Table S8 and 2). Contaminated water, food, and air from sources including battery, pigments, and plastic waste in the EOL ships may be the major source of exposure of Cd (Barua et al. 2018; Basha et al. 2007; Hasan et al. 2020). The adverse health effects of Cd could be associated with kidney damage, lung, and bone after long-term exposure. Urinary Cd is considered a reliable biomarker for chronic exposure to Cd (Ke et al. 2015; Sun et al. 2016; Xiao et al. 2021).
Extensively higher levels of urinary Sb were found in the participant from SWY (p < 0.001) than that of CS, and reference values, where other sites had moderate concentrations compared to CS (Fig. 2, Table S9). Elevated concentrations of Sb (p < 0.0001) were also found in cumulated sites of the exposure groups compared to that of CS (Table S10). Exposure to Sb is a severe threat to human health through the air, water, and food (Ye et al. 2018). Furthermore, occupational exposure to Sb may cause respiratory, cardiovascular, gastrointestinal effects, and carcinogenic to humans (ATSDR 2019). Urinary Sb may be used as an appropriate biomarker for recent and long-term exposures (Makris et al. 2013).
Urinary Hg was significantly higher in the participants from Site-E (p < 0.05) and Site-N (p < 0.05) of exposure groups compared to CS and reference values (Fig. 2, Table S9). Higher concentrations of Hg were also found in cumulated sites of the exposure groups (p < 0.05) (Table S10). There was a minimal effect of demographic characteristics on the Hg levels (Table S8 and 2). However, significantly higher urinary excretion of Hg in environmentally exposed adults may be associated with elevated Hg in the seafood and air caused by shipwrecking activities (Basha et al. 2007; Kakar et al. 2021). Urinary Hg is a valuable biomarker of kidney dysfunction (Jarosińska et al. 2008; WHO 2008).
The levels of Tl were higher in SWY (p < 0.001), Site-E (p < 0.05), Site-S (p < 0.001), and Site-W (p < 0.001) of exposure groups compared to that of CS and other reference values (except, the USA reference population urinary Tl value (Paschal et al. 1998) (Fig. 2, Table S9). Elevated concentrations of Tl (p < 0.0001) were observed in cumulated sites of the exposure groups (Table S10). The World Health Organization (WHO) revealed in 1996 average urinary Tl concentration values between 0.3 and 0.4 μg/L in unexposed populations and between 4.5 and 6 μg/L in exposed subjects with likely health effects (Safety 1996). Our study site’s urinary Tl median concentration was 0.34 to 0.39 μg/L, indicating a health threat to the communities. This highly toxic rare element has carcinogenic effects. Contaminated water, food, air, and soil from sources including nearest cement factories, metal casting, and electronics in SWY or natural may be the major source of exposure of Tl.
The results and discussion of comparatively less significant urinary metals (Cr, Mn, Fe, Ni, Sr, Sn, Cs, Ba, and U) with respect to control/reference values are presented in SI 8. The urinary concentrations of most of the metal/loids were elevated in the permanent residents of the study area than those less than ten years and the control site and, likely due to the shipwrecking activities based on previously published data for soil, water, air, and food samples in the shipwrecking area (Alam et al. 2019; Barua et al. 2018; Basha et al. 2007; Hasan et al. 2020; Hossain et al. 2016).
From the study, the urinary levels of most of the metal/loid in the SWY and the adjoining non-occupational sites of east, north, and south were elevated, likely due to the occupationally exposed participants in the workplace of the yards (Fig. 2, Table S9); the wind aloft flows of contaminants to the latter non-occupational sites dispersed from the shipwrecking activities impacting on the receptors. The wind direction and speed in a year for the SWY zone are presented in Fig. S1, where the prevalent wind blows from the S-SSE-SSW into N-NNE-NNW direction in the wet season (April to October) and from the N-NNE-NE to S-SSW direction in the dry season (November to March). The average wind speed is 16 km/h for the wet seasons and 9 km/h for the dry season (Mark Jacobson et al. 2018; Windrose 2020). The reported findings suggest that wind speed and direction may affect the flow of air particulates, soil dust, and water droplets in the exposure sites (East, north, and south sites).
Metal/loid Concentrations in Drinking Water
The distribution results of the measured metal/loid concentration in drinking water samples from the study and the control area are presented in Table S11. Multiple comparisons of Cd, Sb, Pb, Mn, Ni, As, Se, and Sr concentrations between different sampling locations showed that elemental distribution varies significantly among shipwrecking yards, the surrounding sites from the yards, and the control site. However, the levels of most of them were lower than the standard guideline values of WHO (WHO 2011) and the recommended Bangladesh standard value (Bangladesh 1994) except Mn and As in 63% and 15% water samples, respectively. The highest concentration was found for Mn (Median: 152 μg/L) in the water of the adjoining sites of the SWY compared to the control site (SI 8 for more information on Mn).
Correlation Between Urine and Drinking Water and in Pair of Metal/Loids
Among the urinary samples, Spearman correlation analysis showed that almost all metal/loids are positively correlated at p < 0.01 (Table S13). Furthermore, there was no significant negative correlation in the data (Table S13), suggesting a common origin of the metals. For example, a strong correlation was found between the urinary concentrations of V and Sb (Spearman correlation coefficient, rs = 0.60), Mn (rs = 0.42), Co (rs = 0.44), Sn (rs = 0.48), U (rs = 0.42), and Hg (rs = 0.30). Similarly, urinary Mn concentrations were positively correlated with Fe (rs = 0.68) and Co (rs = 0.33). Urinary Cd concentrations were correlated with Tl (rs = 0.59) and Cs (rs = 0.47). Urinary Cs concentrations were also correlated with Mo (rs = 0.47), Cd (rs = 0.47), and Ni (rs = 0.32) The above findings may indicate the same origin of these elements, which also suggest extreme urinary excretion of these elements could be associated with elevated abundance in the environment due to improper waste management of shipwrecking activities. The relationships for other element pairs are presented in Table S13.
The study also detected that a few metals and metalloids were strongly correlated between the urine and water samples (Table S12 and Fig. 3). For example, significant positive correlations between water and urinary samples were observed for Cd (Pearson’s correlation coefficient, R2 = 0.23, p = 0.03), Tl (R2 = 0.2, p = 0.04), and Mn (R2 = 0.23, p = 0.03) (Fig. 3) suggesting a source of exposure through shipwrecking activities (Barua et al. 2018; Basha et al. 2007; Hasan et al. 2020). However, urinary Mn concentrations were not significantly higher in the exposure sites, except the Site-N site, respectively (SI 8, Fig. S3). No associations were found for other metal/loids of interest (Table S12).
Significant Determinants to the Urinary Metal/Loid Levels
Quantile regression analysis was performed to determine the most significant factors (such as sampling locations and demographic characteristics) influencing the urinary metal/loid concentrations in the local population. The results from the median-based quantile regression are presented in Table 2. Demographic characteristics had no significant influence on urinary excretion of the monitored metal/loids. However, locations of sampling sites with respect to shipwrecking yard were found to be the most significant factors on urinary metal/loid concentrations. Metal/loid concentrations were further analyzed by adjustment with cofounding factors including age, sex, smoking status, fertilizer/pesticide use, betel nut consumption, occupation, and nutritional /body mass status (urinary creatinine concentration) and compared with the control group. The significant positive standardized regression coefficient (β2) values indicated the strong effects of the study sites on urinary concentrations of V (β2 = 0.21–0.41 at p < 0.05, except Site-W with β2 = 0.06 at p = 0.48), Co (β2 = 0.37–0.82 at p < 0.05, except Site-E with β2 = 0.21 at p = 0.20), Sb (β2 = 0.18–0.34 at p < 0.005, except Site-W with β2 = 0.04 at p = 0.49), Hg (β2 = 0.93–1.14 at p < 0.006, with Site-E and Site-N), and Tl (β2 = 0.13–0.24 at p < 0.04) after adjusting several covariates including age, gender, smoking status, fertilizer/pesticide use, betel nut consumption, urinary creatinine concentration/body mass index, and occupation. The influential effects of the study sites on urinary concentrations of Mo (β2 = 30.05–92.86 at p < 0.05) and Cd (β2 = 0.46–1.22 at p < 0.0001) were also found after adjusting several covariates (Table 2).
The shipwrecking yards and their east (Site-E), north (Site-N), and south (Site-S) sites posed significant contributions to the observed urinary concentrations of Cd, Sb, Co, Mo, V, and Tl, likely due to improper waste disposal. Also, the occupational and environmental exposure sites may have been affected by wind-associated dispersion of contaminants. Site-E was comparatively nearer the SWYs (Fig. 1), which may be another reason for the elevated metal/loid concentrations, whereas Site-W was identified as the minor contaminated zone, possibly due to being comparatively far from the shipwrecking yards and negligible wind flow effect on this smaller Island site (Figs. 1 and S1).
Applicability and Implications for Site-Specific Environmental Monitoring and Health
This study adopted a better validated and developed ICP-MS method for measuring the selected urinary metal/loids by comparing matrix-matched and heat digestion methods together with an appropriate hydration variation adjustment. This could be used for the elements biomonitoring and urinary metal/loids biomarker studies due to occupational and environmental exposures.
This study provides important context to metal/loid concentrations reported in previous studies, including environmental soil and sediment in the shipwrecking region. For example, elevated Cd concentrations in soil and sediment (Alam et al. 2019; Barua et al. 2018; Hasan et al. 2020) reflect well with the present study, though elevated levels of other elements in this study are unknown in the previous studies. The study findings may be served to inform a more detailed exposure and health risk assessment to inform management strategy for residents exposed to various environmental matrices and dietary intakes. It also may help to make policy decisions for ensuring sustainable development of the shipwrecking industry around the globe. This biomonitoring approach could be used as a background study for the occupational and environmental exposures of the metal/loids to identify present and future risks that may lead to large-scale health problems in the shipwrecking workers and local peoples at and near shipwrecking yards worldwide.
Study Limitations
Samples were collected at random, and high-quality data were found in the study area with the participants’ oral informed consent. Urine, as opposed to blood, although, is perhaps a less reliable biological indicator of exposure to several metals such as Pb, Se, Cu, and Zn, where such urinary metal concentrations still reflect the current exposure status of the studied groups (ATSDR 2003, 2005, 2012a, 2020; Sanchez et al. 2018; Tanvir et al. 2021). Thus, these elements were not considered in this study. As was not included in exposure estimates because of lack of speciation information. However, total urinary arsenic was only used in this study for comparative assessment of urine dilution correction. Besides, urinary excretion of Cd, Co, and Mo may be more sensitive to reflect the local population's metal/loids exposure levels than blood (CDC 2009; Tanvir et al. 2021; WHO 2001; Zeng et al. 2015). Urine is considered a widely used matrix for biomonitoring studies due to its non-invasiveness and minimal risk affecting the participants (Choe & Gajek 2016; Sanchez et al. 2018; Tanvir et al. 2021). Although the sample size was relatively small, the study afforded meaningful observation of power and bootstrap confidence interval for the metal/loids which was necessarily consistent with the sample size required for representativeness.
The relatively small sample size limitation had made it challenging to identify the definitive determinants of urinary metal/loid levels. However, the statistical analyses suggested many of the elements studied are significant predictors of exposure. Therefore, metal contaminations are implicated due to shipwrecking activities resulting in elevated human exposure to metals.
Conclusions
Significantly higher (p < 0.001) levels of the urinary Cd, Mo, Co, V, Sb, and Tl were observed in the occupationally and environmentally exposed peoples working and/or living at and near a large-scale open beaching shipwrecking yard that has been affected by largely uncontrolled wrecking activities. The significant exposure to these metal/loids in the exposure sites may be largely governed by wind-associated dispersion of contaminants. However, the gender and age of the participants had no significant effect on most of the urinary metal/loid concentrations, while Ni was higher in fertilizer/pesticide users. Moreover, most of the exposed participants (70–80% of shipwrecking workers and 72–75% of the local population) had significantly higher (p < 0.001) urinary levels of Cd and Mo compared to the control, and normal reference levels may be a grave occupational and environmental health concern. For example, urinary Cd levels could be associated with a certain level of body impairment which may be used as a reliable biomarker for exposure to environmental contamination. The elevated urinary concentrations of Cd and Tl were significantly correlated to their concentrations in water. Further studies are warranted to widen the assessments covering food and environmental matrices together with epidemiological investigations. It is prudent that more effective operational and control measures should be taken to minimize occupational and environmental exposures to metals and metalloids.
Data Availability
Data are available in this paper and/or from the corresponding author on reasonable request.
References
Aguilera I, Daponte A, Gil F, Hernández AF, Godoy P, Pla A, Ramos JL (2010) Urinary levels of arsenic and heavy metals in children and adolescents living in the industrialised area of Ria of Huelva (SW Spain). Environ Int 36(6):563–569. https://doi.org/10.1016/j.envint.2010.04.012
Alam I, Barua S, Ishii K, Mizutani S, Hossain MM, Rahman IMM, Hasegawa H (2019) Assessment of health risks associated with potentially toxic element contamination of soil by end-of-life ship dismantling in Bangladesh. (Report). Environ Sci Pollut Res 26(23):24162. https://doi.org/10.1007/s11356-019-05608-x
Al-Rmalli SW, Jenkins RO, Haris PI (2011a) Betel quid chewing as a source of manganese exposure: total daily intake of manganese in a Bangladeshi population. BMC Public Health 11(1):85. https://doi.org/10.1186/1471-2458-11-85
Al-Rmalli SW, Jenkins RO, Haris PI (2011b) Betel quid chewing elevates human exposure to arsenic, cadmium and lead. J Hazard Mater 190(1):69–74. https://doi.org/10.1016/j.jhazmat.2011.02.068
Asante KA, Agusa T, Biney CA, Agyekum WA, Bello M, Otsuka M et al (2012) Multi-trace element levels and arsenic speciation in urine of e-waste recycling workers from Agbogbloshie, Accra in Ghana. Sci Total Environ 424:63–73. https://doi.org/10.1016/j.scitotenv.2012.02.072
ATSDR (2003) Toxicological profile for selenium. In: USA: U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA
ATSDR (2005) Toxicological profile for zinc. In: USA: U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA
ATSDR (2011) Health effects of exposure to substances and carcinogens. Available at www.atsdr.cdc.gov/substances/toxorganlisting.asp?sysid=22. Accessed 01 Jan 2020
ATSDR (2012a) Toxicological profile for manganese. In: USA: U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA
ATSDR (2012b) Toxicological profile for vanadium In: USA: USA: U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA
ATSDR (2019) Toxicological profile for antimony In: USA: USA: U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA
ATSDR (2020) Toxicological profile for lead. In: USA: U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA
Bangladesh MoEaFGotPsRo (1997) The environment conservation rules: standards for water (schedule-3). Available at https://www.elaw.org/system/files/Bangladesh+--+Environmental+Conservation+Rules,+1997.pdf. Accessed 22 Oct 2020
Bangladesh Bureau of Statistics MoP (2014) Population and housing census-2011 (National Report Volume-2: Union Statistics). Available at http://www.bbs.gov.bd/site/page/47856ad0-7e1c-4aab-bd78-892733bc06eb/Population-and-Housing-Census. Accessed 10 June 2020
Bansal N, Hsu C-Y, Zhao S, Whooley MA, Ix JH (2011) relation of body mass index to urinary creatinine excretion rate in patients with coronary heart disease. Am J Cardiol 108(2):179–184. https://doi.org/10.1016/j.amjcard.2011.03.020
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL (2005) Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200. https://doi.org/10.1289/ehp.7337
Barua S, Rahman I, Hossain MM, Begum Z, Alam I, Sawai H et al (2018) Environmental hazards associated with open-beach breaking of end-of-life ships: a review. Environ Sci Pollut Res 25(31):30880–30893. https://doi.org/10.1007/s11356-018-3159-8
Basha S, Gaur PM, Thorat RB, Trivedi RH, Mukhopadhyay SK, Anand N et al (2007) Heavy metal content of suspended particulate matter at world’s largest ship-breaking yard, Alang-Sosiya, India. Water Air Soil Pollut 178(1–4):373–384
Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP (2008) Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 3(2):348–354. https://doi.org/10.2215/cjn.02870707
Berglund M, Lindberg A-L, Rahman M, Yunus M, Grandér M, Lönnerdal B, Vahter M (2011) Gender and age differences in mixed metal exposure and urinary excretion. Environ Res 111(8):1271–1279. https://doi.org/10.1016/j.envres.2011.09.002
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691. https://doi.org/10.1016/j.heliyon.2020.e04691
Burch BD (2012) Nonparametric bootstrap confidence intervals for variance components applied to interlaboratory comparisons. J Agric Biol Environ Stat 17(2):228–245. https://doi.org/10.1007/s13253-012-0087-9
CDC (2009) Fourth national report on human exposure to environmental chemicals. Available at https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf. Accessed 1 Jan 2022
Chakraborti D, Rahman MM, Das B, Murrill M, Dey S, Chandra Mukherjee S et al (2010) Status of groundwater arsenic contamination in Bangladesh: a 14-year study report. Water Res 44(19):5789–5802. https://doi.org/10.1016/j.watres.2010.06.051
Choe K-Y, Gajek R (2016) Determination of trace elements in human urine by ICP-MS using sodium chloride as a matrix-matching component in calibration. Anal Methods 8(37):6754–6763. https://doi.org/10.1039/C6AY01877G
Ciminelli VST, Gasparon M, Ng JC, Silva GC, Caldeira CL (2017) Dietary arsenic exposure in Brazil: the contribution of rice and beans. Chemosphere 168:996–1003. https://doi.org/10.1016/j.chemosphere.2016.10.111
Concha G, Broberg K, Grandér M, Cardozo A, Palm B, Vahter M (2010) High-level exposure to lithium, boron, cesium, and arsenic via drinking water in the andes of Northern Argentina. Environ Sci Technol 44(17):6875–6880. https://doi.org/10.1021/es1010384
Creed JTB, Brockhoff CA, Martin TD (1994) Determination of trace elements in waters and wastes by Inductively Coupled Plasma-Mass spectrometry (method 200.8, Revision 5.4). US Environmental Protection Agency, Cincinnati, Ohio 45268. https://www.epa.gov/sites/default/files/2015-06/documents/epa-200.8.pdf. Accessed 12 Dec 2020
Deshpande PC, Tilwankar AK, Asolekar SR (2012) A novel approach to estimating potential maximum heavy metal exposure to ship recycling yard workers in Alang, India. Sci Total Environ 438:304–311. https://doi.org/10.1016/j.scitotenv.2012.08.048
Ebrahimi Yazdanabad T, Emtyazjoo M, Ghavam Mostafavi P, Sahebi Z (2014) Evaluation of copper and vanadium concentration in the soft tissue of Tylosurus crocodilus in Bahregan region, Persian Gulf. Chem Spec Bioavail 26(2):92–98. https://doi.org/10.3184/095422914X13952273632308
Freire BM, Pedron T, Lange CN, Sanches LR, Barcelos GRM, Filho WRP, Batista BL (2018) Calibration for the determination of 19 trace elements in serum and urine. Toxicol Environ Chem 100(4):395–412. https://doi.org/10.1080/02772248.2018.1537397
Gunbeyaz SA, Kurt RE, Baumler R (2019) A study on evaluating the status of current occupational training in the ship recycling industry in Bangladesh. WMU J Marit Aff 18(1):41–59. https://doi.org/10.1007/s13437-019-00164-0
Hasan AB, Reza AHMS, Kabir S, Siddique MAB, Ahsan MA, Akbor MA (2020) Accumulation and distribution of heavy metals in soil and food crops around the ship breaking area in southern Bangladesh and associated health risk assessment. SN Appl Sci 2(2):155. https://doi.org/10.1007/s42452-019-1933-y
Heitland P, Köster HD (2004) Fast, simple and reliable routine determination of 23 elements in urine by ICP-MS. J Anal at Spectrom 19(12):1552–1558. https://doi.org/10.1039/B410630J
Hossain MM, Islam M (2006) Ship breaking activities and its impact on the coastal zone of Chittagong, Bangladesh: towards sustainable management. An YPSA publication, Chittagong
Hossain MS, Fakhruddin ANM, Chowdhury MAZ, Gan SH (2016) Impact of ship-breaking activities on the coastal environment of Bangladesh and a management system for its sustainability. Environ Sci Policy 60:84–94. https://doi.org/10.1016/j.envsci.2016.03.005
ILO (2004) Safety and health in ship breaking: guidelines for Asian countries and Turkey, interregional tripartite meeting of experts on safety and health in shipbreaking for selected Asian countries and Turkey, Bangkok, ISBN 92-2115289-8
ILO (2015) Ship-breaking: a hazardous work. Available at https://www.ilo.org/global/topics/safety-and-health-at-work/areasofwork/hazardous-work/WCMS_356543/lang--en/index.htm. Accessed 16 Aug 2020
Islam MN, Hossain MA, Ahmed MJ (2013) Physicochemical assessment of water pollutants due to the ship breaking activities and its impact on the coastal environment of Chittagong-Bangladesh. Eur Chem Bull 2(12):1053–1059
Jarosińska D, Horvat M, Sällsten G, Mazzolai B, Dąbkowska B, Prokopowicz A et al (2008) Urinary mercury and biomarkers of early renal dysfunction in environmentally and occupationally exposed adults: a three-country study. Environ Res 108(2):224–232. https://doi.org/10.1016/j.envres.2008.06.005
Kakar A, Liem-Nguyen V, Mahmood Q, Jonsson S (2021) Elevated concentrations of mercury and methylmercury in the Gadani shipbreaking area, Pakistan. Mar Pollut Bullet 165:112048. https://doi.org/10.1016/j.marpolbul.2021.112048
Ke S, Cheng X-Y, Li H, Jia W-J, Zhang J-Y, Luo H-F et al (2015) Body burden of cadmium and its related factors: a large-scale survey in China. Sci Total Environ 511:649–654. https://doi.org/10.1016/j.scitotenv.2015.01.014
Kettelarij J, Midander K, Lidén C, Bottai M, Julander A (2018) Neglected exposure route: cobalt on skin and its associations with urinary cobalt levels. Occup Environ Med 75(11):837–842. https://doi.org/10.1136/oemed-2018-105099
Khlifi R, Olmedo P, Gil F, Feki-Tounsi M, Hammami B, Rebai A, Hamza-Chaffai A (2014) Biomonitoring of cadmium, chromium, nickel and arsenic in general population living near mining and active industrial areas in Southern Tunisia. Environ Monit Assess 186(2):761–779. https://doi.org/10.1007/s10661-013-3415-9
Kumar S, Prasad S, Yadav K, Shrivastava M, Gupta N, Nagar S et al (2019) Hazardous heavy metals contamination of vegetables and food chain: role of sustainable remediation approaches—a review. Environ Res. https://doi.org/10.1016/j.envres.2019.108792
Makris KC, Andra SS, Herrick L, Christophi CA, Snyder SA, Hauser R (2013) Association of drinking-water source and use characteristics with urinary antimony concentrations. J Eposure Sci Environ Epidemiol 23(2):120–127. https://doi.org/10.1038/jes.2012.104
Mark Jacobson CD, Jimenez T, O’Neill B, Laboratory NRE (2018) Assessing the Wind Energy Potential in Bangladesh: Enabling Wind Energy Development with Data Products. https://www.nrel.gov/docs/fy18osti/71077.pdf. Accessed 16 Oct 2020
Middleton DRS, Watts MJ, Polya DA (2019) A comparative assessment of dilution correction methods for spot urinary analyte concentrations in a UK population exposed to arsenic in drinking water. Environ Int 130:104721. https://doi.org/10.1016/j.envint.2019.03.069
Ministry of Industries (2011) The ship breaking and ship recycling rules in Bangladesh, Act 13, 87 of The Factories Act ,1965
Nicolae A, Ames H, Quiñonez C (2013) Dental amalgam and urinary mercury concentrations: a descriptive study. BMC Oral Health 13:44–44. https://doi.org/10.1186/1472-6831-13-44
Paschal DC, Ting BG, Morrow JC, Pirkle JL, Jackson RJ, Sampson EJ et al (1998) Trace metals in urine of United States residents: reference range concentrations. Environ Res 76(1):53–59. https://doi.org/10.1006/enrs.1997.3793
Platform NS (2019) Annual impact report, 2018–19.11, B - 1060 Brussels. Available at https://www.shipbreakingplatform.org/wp-content/uploads/2018/07/Annual-Report-2017-Final-Spreads.pdf, Accessed 18 June 2020
Protano C, Astolfi ML, Canepari S, Vitali M (2016) Urinary levels of trace elements among primary school-aged children from Italy: The contribution of smoking habits of family members. Sci Total Environ 557–558:378–385. https://doi.org/10.1016/j.scitotenv.2016.03.073
Safety WHOIPoC (1996) International program on chemical safety: thallium. Environmental Health Criteria 182. Available at https://inchem.org/documents/ehc/ehc/ehc182.htm. Accessed 21 Oct 2020
Sanchez TR, Slavkovich V, LoIacono N, van Geen A, Ellis T, Chillrud SN et al (2018) Urinary metals and metal mixtures in Bangladesh: exploring environmental sources in the Health Effects of Arsenic Longitudinal Study (HEALS). Environ Int 121:852–860. https://doi.org/10.1016/j.envint.2018.10.031
Sarraf M, Stuer-Lauridsen F, Dyoulgerov M, Bloch R, Wingfield S, Watkinson R (2010) The ship breaking and recycling industry in Bangladesh and Pakistan, Report No 58275-SAS (Report No 58275-SAS)
Smedley PL, Kinniburgh DG (2017) Molybdenum in natural waters: a review of occurrence, distributions and controls. Appl Geochem 84:387–432. https://doi.org/10.1016/j.apgeochem.2017.05.008
Sujauddin M, Koide R, Komatsu T, Hossain M, Tokoro C, Murakami S (2015) Characterization of ship breaking industry in Bangladesh. J Mater Cycles Waste Manag 17(1):72–83. https://doi.org/10.1007/s10163-013-0224-8
Sujauddin M, Koide R, Komatsu T, Hossain MM, Tokoro C, Murakami S (2017) Ship breaking and the steel industry in Bangladesh: a material flow perspective. J Ind Ecol 21(1):191–203. https://doi.org/10.1111/jiec.12423
Sun H, Wang D, Zhou Z, Ding Z, Chen X, Xu Y et al (2016) Association of cadmium in urine and blood with age in a general population with low environmental exposure. Chemosphere 156:392–397. https://doi.org/10.1016/j.chemosphere.2016.05.013
Tanvir EM, Whitfield KM, Ng JC, Shaw PN (2020) Development and validation of an ICP-MS method and its application to determine multiple trace elements in small volumes of whole blood and plasma. J Anal Toxicol 44:1036–1046. https://doi.org/10.1093/jat/bkaa033
Tanvir EM, Mahmood S, Islam MN, Khatun M, Afroz R, Islam SS et al (2021) Environmental exposure to metals and metalloids in primary school-aged children living in industrialised areas of eastern south asian Megacity Dhaka, Bangladesh. Expo Health. https://doi.org/10.1007/s12403-021-00443-3
WHO (2001) Arsenic and arsenic compounds, 2nd edn. Environmental health criteria 224. Available at http://www.inchem.org/documents/ehc/ehc/ehc224.htm. Accessed 1 Jan 2022
WHO (2004) Manganese in drinking-water: background document for development of WHO guidelines for drinking-water quality. Available at https://apps.who.int/iris/bitstream/handle/10665/75376/WHO_SDE_WSH_03.04_104_eng.pdf. Accessed 21 Oct 2020
WHO (2008) Guidance for identifying populations at risk from mercury exposure. Geneva, Switzerland. Available at https://www.who.int/foodsafety/publications/risk-mercury-exposure/en/. Accessed 31 May 2020
WHO (2011) Guidelines for drinking water quality. Available at https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng. Accessed 22 Oct 2020
Windrose and meteoblue (2020) Available at https://www.meteoblue.com/en/weather/historyclimate/climatemodelled/chittagong_bangladesh_1205733. Accessed 01 Jan 2020
Xiao L, Zhu C, Yang S, Zhou M, Wang B, Wang X et al (2021) Assessment of the variability of urinary cadmium for general adults. Chemosphere 269:128752. https://doi.org/10.1016/j.chemosphere.2020.128752
Ye L, Qiu S, Li X, Jiang Y, Jing C (2018) Antimony exposure and speciation in human biomarkers near an active mining area in Hunan, China. Sci Total Environ 640–641:1–8. https://doi.org/10.1016/j.scitotenv.2018.05.267
Zeng Q, Feng W, Zhou B, Wang Y-X, He X-S, Yang P et al (2015) Urinary metal concentrations in relation to semen quality: a cross-sectional study in China. Environ Sci Technol 49(8):5052–5059. https://doi.org/10.1021/es5053478
Acknowledgements
We acknowledge the postgraduate students, Biomaterials Research Laboratory, Department of Applied Chemistry and Chemical Engineering (ACCE), University of Chittagong, Chittagong-4331, Bangladesh, for their cordial supports in urine and water sampling and obtaining demographic information data from the residents. QAEHS is a partnership between Queensland Health and the University of Queensland.
Disclaimer
The views expressed in this publication are those of the authors and do not necessarily reflect the opinions or policies of the shipwrecking yards and the government of Bangladesh.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Md. Nazrul Islam is supported by an Australian Government Research Training Program (RTP) Scholarship and Queensland Alliance for Environmental Health Sciences (QAEHS) from the University of Queensland, Brisbane, QLD 4072, Australia.
Author information
Authors and Affiliations
Contributions
MNI: Conceptualization, method development, measurement, analysis of results, and the draft manuscript preparation. SG: Sampling, writing, and editing. EMT: Methodology, statistical analysis, writing, and editing. MAHR: Sampling, writing, and editing. NS: Statistical analysis, writing, and editing. CP: Conceptualization, planning, writing, and editing. JCN: Conceptualization, planning, supervision, and laboratory resources, obtaining funds, writing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests or personal relationships that would have appeared to affect the work reported in this paper.
Ethical Approval
The study protocols were approved by the Ethics Committee, Directorate of Research and Extension, Chittagong Veterinary and Animal Sciences University, Chittagong (Chattogram), Bangladesh (Memo no.-CVASU/Dir(R&E) EC/2020/191/3). All participants provided oral informed consent.
Consent for Publication
All authors have given their consent to publish this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Islam, M.N., Ganguli, S., Tanvir, E.M. et al. Human Exposure Assessment of Mixed Metal/Loids at and Near Mega-Scale Open Beaching Shipwrecking Activities in Bangladesh. Expo Health 15, 69–84 (2023). https://doi.org/10.1007/s12403-022-00477-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-022-00477-1