Abstract
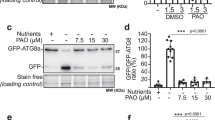
In plant cells, autophagy is required for efficient recycling of cytoplasmic macromolecules in vacuoles. It was previously shown that autophagy-deficient mutants also exhibited hypersensitivity to various abiotic stresses, such as salt, osmotic changes, heat, drought, and oxidative damage. However, it has not been clearly determined whether autophagy is induced or inhibited by these environmental stressors. Using the GFP-ATG8 (green fluorescent protein fused to AUTOPHAGY-RELATED PROTEIN 8) processing assay and confocal microscopy, we assessed autophagic flux of Arabidopsis seedlings exposed to salt stress. Treatment with 150 mM NaCl resulted in an increase in the processing of GFP-ATG8. Notably, the effects of concanamycin A, an inhibitor of vacuolar proton pumps, on GFP-ATG8 processing indicated that the apparent increase in GFP-ATG8 processing by salt-induced stress was due to inefficient vacuolar degradation of the GFP moiety processed from GFP-ATG8. Salt and osmotic stresses did not increase the abundance of autophagic vesicles in the root cells. Although NaCl, KCl, and mannitol did not greatly inhibit the vacuolar trafficking of GFP-ATG8, LiCl partially inhibited autophagy. These data indicated that NaCl stress neither increases nor substantially inhibits autophagic flux. Our work illustrates the importance of autophagic flux analysis to assess the effect of abiotic stresses on plant autophagy.
Similar content being viewed by others
References
Baral A, Irani NG, Fujimoto M, Nakano A, Mayor S, Mathew MK (2015) Salt-induced remodeling of spatially restricted clathrinindependent endocytic pathways in Arabidopsis root. Plant Cell 27:1297–1315
Bassham DC (2015) Methods for analysis of autophagy in plants. Methods 75:181–188
Blumwald E, Poole RJ (1985) Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol 78: 163–167
Chen L, Liao B, Qi H, Xie LJ, Huang L, Tan WJ, Zhai N, Yuan LB, Zhou Y, Yu LJ, Chen QF, Shu W, Xiao S (2015) Autophagy contributes to regulation of the hypoxia response during submergence in Arabidopsis thaliana. Autophagy 11:2233–2246
Chung T, Phillips AR, Vierstra RD (2010) ATG8 lipidation and ATG8-mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A AND ATG12B loci. Plant J 62:483–493
Chung T, Suttangkakul A, Vierstra RD (2009) The ATG autophagic conjugation system in maize: ATG transcripts and abundance of the ATG8-lipid adduct are regulated by development and nutrient availability. Plant Physiol 149:220–234
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Contento AL, Xiong Y, Bassham DC (2005) Visualization of autophagy in Arabidopsis using the fluorescent dye monodansylcadaverine and a GFP-AtATG8e fusion protein. Plant J 42:598–608
Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD (2002) The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J Biol Chem 277:33105–33114
Gillaspy GE, Keddie JS, Oda K, Gruissem W (1995) Plant inositol monophosphatase is a lithium-sensitive enzyme encoded by a multigene family. Plant Cell 7:2175–2185
Hamaji K, Nagira M, Yoshida K, Ohnishi M, Oda Y, Uemura T, Goh T, Sato MH, Morita MT, Tasaka M, Hasezawa S, Nakano A, Hara-Nishimura I, Maeshima M, Fukaki H, Mimura T (2009) Dynamic aspects of ion accumulation by vesicle traffic under salt stress in Arabidopsis. Plant Cell Physiol 50:2023–2033
Kim J, Lee H, Lee HN, Kim SH, Shin KD, Chung T (2013) Autophagy-Related Proteins Are Required for Degradation of Peroxisomes in Arabidopsis Hypocotyls during Seedling Growth. Plant Cell 25:4956–4966
Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen G, Levine A (2006) Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci U S A 103:18008–18013
Li F, Chung T, and Vierstra RD (2014) AUTOPHAGY-RELATED (ATG)11 plays a critical role in general autophagy and senescenceinduced mitophagy in Arabidopsis. Plant Cell 26:788–807
Li F, Vierstra RD (2012) Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci 17: 526–537
Liu Y, Xiong Y, Bassham DC (2009) Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 5:954–963
Merkulova EA, Guiboileau A, Naya L, Masclaux-Daubresse C, Yoshimoto K (2014) Assessment and optimization of autophagy monitoring methods in Arabidopsis roots indicate direct fusion of autophagosomes with vacuoles. Plant Cell Physiol 55:715–726
Michaeli S, Honig A, Levanony H, Peled-Zehavi H, Galili G (2014) Arabidopsis ATG8-INTERACTING PROTEIN1 is involved in autophagy-dependent vesicular trafficking of plastid proteins to the vacuole. Plant Cell 26:4084–4101
Sakuraba Y, Lee SH, Kim YS, Park OK, Hortensteiner S, Paek NC (2014) Delayed degradation of chlorophylls and photosynthetic proteins in Arabidopsis autophagy mutants during stress-induced leaf yellowing. J Exp Bot 65:3915–3925
Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170:1101–1111
Sarkar S, Krishna G, Imarisio S, Saiki S, O'Kane CJ, Rubinsztein DC (2008) A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin. Hum Mol Genet 17:170–178
Shin KD, Lee HN, Chung T (2014) A revised assay for monitoring autophagic flux in Arabidopsis thaliana reveals involvement of AUTOPHAGY-RELATED9 in autophagy. Mol Cells 37:399–405
Suttangkakul A, Li F, Chung T, Vierstra RD (2011) The ATG1/ ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. Plant Cell 23:3761–3779
Xiong Y, Contento AL, Nguyen PQ, Bassham DC (2007) Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol 143:291–299
Yoshimoto K, Hanaoka H, Sato S, Kato T, Tabata S, Noda T, Ohsumi Y (2004) Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 16:2967–2983
Zhai Y, Guo M, Wang H, Lu J, Liu J, Zhang C, Gong Z, Lu M (2016) Autophagy, a Conserved Mechanism for Protein Degradation, Responds to Heat, and Other Abiotic Stresses in Capsicum annuum L. Front Plant Sci 7:131
Zhao J, Peng P, Schmitz RJ, Decker AD, Tax FE, Li J (2002) Two putative BIN2 substrates are nuclear components of brassinosteroid signaling. Plant Physiol 130:1221–1229
Zhou J, Wang J, Cheng Y, Chi YJ, Fan B, Yu JQ, Chen Z (2013) NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet 9: e1003196
Zhuang X, Wang H, Lam SK, Gao C, Wang X, Cai Y, Jiang L (2013) A BAR-domain protein SH3P2, which binds to phosphatidylinositol 3-phosphate and ATG8, regulates autophagosome formation in Arabidopsis. Plant Cell 25:4596–4615
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, H., Kim, J.H., Shin, K.D. et al. Autophagic flux analysis of Arabidopsis seedlings exposed to salt stress. J. Plant Biol. 60, 199–206 (2017). https://doi.org/10.1007/s12374-016-0448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-016-0448-y