Abstract
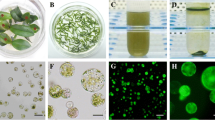
The transient gene expression of protoplasts in plant is a considerable tool for gene functional research that has been widely used in gene analysis and functional characterization. Therefore, the objectives of this study were to develop a protocol for the isolation and purification of sugarcane protoplasts (Saccharum spp. hybrids), conduct transient PEG-mediated protoplast transfection with D27, and localize the D27 protein in sugarcane protoplasts. Total yield and viability of protoplasts were optimized for enzyme combination, mannitol concentration, and duration and temperature of enzymatic hydrolysis. High production of intact protoplasts (10.94 × 106 protoplasts g−1 FW) and a survival rate of > 80.0% was achieved through enzymatic hydrolysis at constant temperature of 28 °C, 60–70 rpm min−1 for 8 h in a solution containing 2.0% cellulase R-10, 0.5% macerozyme R-10, 0.6% pectolyase Y-23, 20 mM 2-(N-morpholine) ethanesulfonic acid (MES), 20 mM KCl, and 400 mM mannitol (pH 5.7). Using GFP as the reporter gene, the protoplasts were transformed most efficiently with 25% PEG 4000 for 25 min and the ScD27 protein was localized in the chloroplasts. The localization of ScD27 protein in sugarcane protoplast demonstrated that the newly developed protocol was functionally effective. This optimized sugarcane protoplast isolation, purification, and transient expression protocol lays a foundation for future molecular biology research in sugarcane.








Similar content being viewed by others
References
Andersson, M., H. Turesson, A. Nicolia, A.S. Falt, M. Samuelsson, and P. Hofvander. 2017. Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Reports 36(1): 117–128.
Cao, Y., H. Li, A.Q. Pham, and G. Stacey. 2016. An improved transient expression system using arabidopsis protoplasts. Current Protocols in Plant Biology 1(2): 285–291.
Chen, W.H., K.M. Gartland, M.R. Davey, R. Sotak, J.S. Gartland, B.J. Mulligan, J.B. Power, and E.C. Cocking. 1987. Transformation of sugarcane protoplasts by direct uptake of a selectable chimaeric gene. Plant Cell Reports 6(4): 297–301.
Chen, W.H., M.R. Davey, J.B. Power, and E.C. Cocking. 1988. Sugarcane protoplasts: Factors affecting division and plant regeneration. Plant Cell Reports 7(5): 344–347.
Chen, J., Q. Yi, Q. Song, Y. Gu, J. Zhang, Y. Hu, H. Liu, Y. Liu, G. Yu, and Y. Huang. 2015. A highly efficient maize nucellus protoplast system for transient gene expression and studying programmed cell death-related processes. Plant Cell Reports 34(7): 1239–1251.
Figueiredo, J.F., P. Romer, T. Lahaye, J.H. Graham, F.F. White, and J.B. Jones. 2011. Agrobacterium-mediated transient expression in citrus leaves: a rapid tool for gene expression and functional gene assay. Plant Cell Reports 30(7): 1339–1345.
Flematti, G.R., A. Scaffidi, M.T. Waters, and S.M. Smith. 2016. Stereospecificity in strigolactone biosynthesis and perception. Planta 243(6): 1361–1373.
Gomez-Roldan, V., S. Fermas, P.B. Brewer, V. Puech-Pages, E.A. Dun, J.P. Pillot, F. Letisse, R. Matusova, S. Danoun, J.C. Portais, H. Bouwmeester, G. Becard, C.A. Beveridge, C. Rameau, and S.F. Rochange. 2008. Strigolactone inhibition of shoot branching. Nature 455(7210): 189–194.
He, F., S. Chen, Y. Ning, and G.L. Wang. 2016. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Current Protocols in Plant Biology 1(2): 373–383.
Huang, H., Z. Wang, J. Cheng, W. Zhao, X. Li, H. Wang, Z. Zhang, and X. Sui. 2013. An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Scientia Horticulturae 150: 206–212.
Im, J.H., and S.D. Yoo. 2014. Transient expression in Arabidopsis leaf mesophyll protoplast system for cell-based functional analysis of MAPK cascades signaling. Methods in Molecular Biology 1171: 3–12.
Kim, N., S.J. Moon, M.K. Min, E.H. Choi, J.A. Kim, E.Y. Koh, I. Yoon, M.O. Byun, S.D. Yoo, and B.G. Kim. 2015. Functional characterization and reconstitution of ABA signaling components using transient gene expression in rice protoplasts. Frontiers in Plant Science 6: 614.
Larkin, P.J. 1976. Purification and viability determinations of plant protoplasts. Planta 128(3): 213–216.
Lin, W. 1980. Corn root protoplasts: Isolation and general characterization of ion transPORT. Plant Physiology 66(4): 550–554.
Lin, H., R. Wang, Q. Qian, M. Yan, X. Meng, Z. Fu, C. Yan, B. Jiang, Z. Su, J. Li, and Y. Wang. 2009. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21(5): 1512–1525.
Nanjareddy, K., M. Arthikala, L. Blanco, E.S. Arellano, and M. Lara. 2016. Protoplast isolation, transient transformation of leaf mesophyll protoplasts and improved Agrobacterium-mediated leaf disc infiltration of Phaseolus vulgaris: Tools for rapid gene expression analysis. BMC Biotechnology 16(1): 53.
Ortiz-Ramirez, C., E.D. Arevalo, X. Xu, D.P. Jackson, and K.D. Birnbaum. 2018. An efficient cell sorting protocol for maize protoplasts. Current Protocols in Plant Biology 3(3): e20072.
Rose, R.J. 2019. Somatic embryogenesis in the medicago truncatula model: Cellular and molecular mechanisms. Frontiers in Plant Science 10: 267.
Sahab, S., M.J. Hayden, J. Mason, and G. Spangenberg. 2019. Mesophyll protoplasts and peg-mediated transfections: Transient assays and generation of stable transgenic canola plants. Methods in Molecular Biology 1864: 131–152.
Shen, J., J. Fu, J. Ma, X. Wang, C. Gao, C. Zhuang, J. Wan, and L. Jiang. 2014. Isolation, culture, and transient transformation of plant protoplasts. Current Protocols in Plant Biology 63: 2–8.
Shen, Y., D. Meng, K. McGrouther, J. Zhang, and L. Cheng. 2017. Efficient isolation of Magnolia protoplasts and the application to subcellular localization of MdeHSF1. Plant Methods 13: 44.
Shu, Y., L. Huang, M. Chen, Y. Tao, Z. Wang, and H. Ma. 2017. Establishment and optimization of systems for protoplasts isolation of soybean and chickpea that used in subcellular location. Sheng Wu Gong Cheng Xue Bao 33(6): 976–985.
Smith, G.R., R. Ford, M.J. Frenkel, D.D. Shukla, and J.L. Dale. 1992. Transient expression of the coat protein of sugarcane mosaic virus in sugarcane protoplasts and expression in Escherichia coli. Archives of Virology 125(1–4): 15–23.
Sun, B., F. Zhang, N. Xiao, M. Jiang, Q. Yuan, S. Xue, H. Miao, Q. Chen, M. Li, X. Wang, Q. Wang, and H. Tang. 2018. An efficient mesophyll protoplast isolation, purification and PEG-mediated transient gene expression for subcellular localization in Chinese kale. Scientia Horticulturae 241: 187–193.
Titouh, K., N. Boufis, and L. Khelifi. 2017. Microcalli induction in protoplasts isolated from embryogenic callus of date palm. Methods in Molecular Biology 1637: 227–237.
Umehara, M., A. Hanada, S. Yoshida, K. Akiyama, T. Arite, N. Takeda-Kamiya, H. Magome, Y. Kamiya, K. Shirasu, K. Yoneyama, J. Kyozuka, and S. Yamaguchi. 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455(7210): 195–200.
Vogel, J.T., M.H. Walter, P. Giavalisco, A. Lytovchenko, W. Kohlen, T. Charnikhova, A.J. Simkin, C. Goulet, D. Strack, H.J. Bouwmeester, A.R. Fernie, and H.J. Klee. 2010. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. The Plant Journal 61(2): 300–311.
Wang, J., T. Zhao, B. Yang, W. Wang, C. Feng, X. Feng, L. Shen, and S. Zhang. 2019. Characteristics, expression pattern and intracellular localisation of sugarcane cytoplasmic hexokinase gene ShHXK8. Sugar Tech 21(6): 909–916.
Waters, M.T., P.B. Brewer, J.D. Bussell, S.M. Smith, and C.A. Beveridge. 2012. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiology 159(3): 1073–1085.
Waters, M.T., C. Gutjahr, T. Bennett, and D.C. Nelson. 2017. Strigolactone signaling and evolution. Annual Review of Plant Biology 68: 291–322.
Wen, C., Q. Zhao, J. Nie, G. Liu, L. Shen, C. Cheng, L. Xi, N. Ma, and L. Zhao. 2016. Physiological controls of chrysanthemum DgD27 gene expression in regulation of shoot branching. Plant Cell Reports 35(5): 1053–1070.
Wu, Z.D., X.L. Liu, J.Y. Liu, F.G. Zan, X.J. Li, H.B. Liu, X.Q. Lin, X.K. Chen, H.S. Su, P.F. Zhao, and C.W. Wu. 2017a. Cloning and expression analysis of key gene ScD27 in strigolactones biosynthesis pathway. Acta Agronomica Sinica 48: 1554–1559.
Wu, J.Z., Q. Liu, X.S. Geng, K.M. Li, L.J. Luo, and J.P. Liu. 2017b. Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz). BMC Biotechnology 17(1): 29.
Xiong, L., C. Li, H. Li, X. Lyu, T. Zhao, J. Liu, Z. Zuo, and B. Liu. 2019. A transient expression system in soybean mesophyll protoplasts reveals the formation of cytoplasmic GmCRY1 photobody-like structures. Science China Life Sciences 62: 1070–1077.
Yoo, S.D., Y.H. Cho, and J. Sheen. 2007. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nature Protocols 2(7): 1565–1572.
Yu, G., Q. Cheng, Z. Xie, B. Xu, B. Huang, and B. Zhao. 2017. An efficient protocol for perennial ryegrass mesophyll protoplast isolation and transformation, and its application on interaction study between LpNOL and LpNYC1. Plant Methods 13: 46.
Zhang, Y., J. Su, S. Duan, Y. Ao, J. Dai, J. Liu, P. Wang, Y. Li, B. Liu, D. Feng, J. Wang, and H. Wang. 2011. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7(1): 30.
Acknowledgments
This study was supported by Fund for the National Natural Science Foundation of China (31860405); Earmarked Fund for China Agriculture Research System (CARS-170101); Provincial Innovation Team of Sugarcane Germplasm Innovation and New Variety Breeding of Yunnan Academy of Agricultural Sciences (2019HC013); Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630052017020-4); The Applied Basic Research Projects in Yunnan Province (2016FB071); Applied Basic Research Projects of Yunnan Academy of Agricultural Sciences (YJM201705); Overseas Top Talents Project “Sugarcane genetic improvement and extension”; Yunnan Provincial Science and Technology Cooperation Program, China (Yunnan) -Sri Lanka Sugarcane International Joint Research Center (2018IA076); The Key Research and Development Program of Yunnan Province, the Joint Research and Development Center of Sugarcane Variety Improvement in South and Southeast Asia (2019IB008).
Author information
Authors and Affiliations
Contributions
CWW and FGZ, ZDW, XKC conceived and designed the experiments. ZDW, FGZ, XH, and ZYL performed the experiments. FGZ, ZDW, and XKC result analysis, and manuscript drafting. YBP, XKC, and DMB revised the manuscript. All authors have read and approved the final manuscript. XKC, JYL, LPZ, LY, YZ, XLL, HMX, KY, JZ, PFZ, and WQ participated in experimental work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Zd., Hu, X., Zan, Fg. et al. Subcellular Localization of the D27 Protein in Sugarcane (Saccharum spp. Hybrids) Using an Optimized Protoplast Isolation, Purification, and Transient Gene Expression Protocol. Sugar Tech 23, 316–325 (2021). https://doi.org/10.1007/s12355-020-00879-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-020-00879-y