Abstract
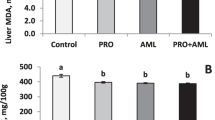
In the field of biology, free radicals which are derived from the incomplete reduction of oxygen take on great importance; they belong to the so called reactive oxygen species, whose production in the organism is an inevitable consequence of various external or internal factors to which it is exposed. Once free radicals are generated they are often capable of giving rise to chain reactions. A lot of biological molecules are susceptible to the attack by free radicals including lipids, proteins, carbohydrates and nucleic acids. Molecular alterations caused by the radical reactions have been frequently studied and are considered as pathogenetically main passages in the development of many diseases and ageing. In order to face a radical attack, living organisms have developed several biological defensive systems against it: the main ones are represented by anti oxidizing molecules and by enzymatic anti oxidizing systems. Among the various defence systems, glutathione stands out as the principal guarantor of homoeostatic intra-cellular oxidation–reduction. One of glutathione’s most important functions is to act as cysteine “tank”; this amino acid is extremely unstable in the extra-cellular environment and it rapidly auto-oxidates. Whey proteins (WP) are particularly rich in cysteine (cys) and in glutamine (glu) and therefore potentially capable of increasing the organism’s antioxidant defences. It is thought that the principal mechanism which allows WPs to exert their properties is through the contribution of cys and glu, which is rich in these proteins and is used intra-cellularly for the synthesis of glutathione. A diet based on milk serum proteins which supplies a superior quantity of cys, allows for a greater synthesis of hepatic glutathione in oxidative stress conditions. The use of ultra-filtrated WP could represent a useful tool in the control of oxidative stress in numerous pathological situations.
Similar content being viewed by others
References
Harman D (1992) Free radical theory of ageing. Mutat Res 275:257–266
Halliwell B (1989) Tell me about free radicals, doctor: a review. J R Soc Med 82:747–752
Purkayastha S, Milligan JR, Bernhard WA (2006) The role of hydration in the distribution of free radical trapping in directly ionized DNA. Radiat Res 166:1–8
Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP (2002) In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J 16:1713–1720
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230
Sanz A, Caro P, Sanchez JG, Barja G (2006) Effect of lipid restriction on mitochondrial free radical production and oxidative DNA damage. Ann NY Acad Sci 1067:200–209
Kennedy CH, Mason RP (1990) A reexamination of the cytochrome P-450-catalyzed free radical production from a dihydropyridine. Evidence of trace transition metal catalysis. J Biol Chem 265:11425–11428
Faivre B, Menu P, Labrude P, Vigneron C (1998) Hemoglobin autooxidation/oxidation mechanisms and methemoglobin prevention or reduction processes in the bloodstream. Literature review and outline of autooxidation reaction. Artif Cells Blood Substit Immobil Biotechnol 26:17–26
Hunt JV, Dean RT, Wolff SP (1988) Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J 256:205–212
Miyata T, Inagi R, Asahi K, Yamada Y, Horie K, Sakai H, Uchida K, Kurokawa K (1998) Generation of protein carbonyls by glycoxidation and lipoxidation reactions with autoxidation products of ascorbic acid and polyunsaturated fatty acids. FEBS Lett 437:24–28
Sevanian A, Ursini F (2000) Lipid peroxidation in membranes and low-density lipoproteins: similarities and differences. Free Radic Biol Med 29:306–311
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Gieseg S, Duggan S, Gebicki JM (2000) Peroxidation of proteins before lipids in U937 cells exposed to peroxyl radicals. Biochem J 350(Pt 1):215–218
Gebicki S, Gebicki JM (1993) Formation of peroxides in amino acids and proteins exposed to oxygen free radicals. Biochem J 289(Pt 3):743–749
Neuzil J, Gebicki JM, Stocker R (1993) Radical-induced chain oxidation of proteins and its inhibition by chain-breaking antioxidants. Biochem J 293(Pt 3):601–606
Davies MJ, Fu S, Wang H, Dean RT (1999) Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic Biol Med 27:1151–1163
Lu SC (1999) Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 13:1169–1183
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
de la Asuncion JG, Millan A, Pla R, Bruseghini L, Esteras A, Pallardo FV, Sastre J, Vina J (1996) Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J 10:333–338
Griffith OW (1999) Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27:922–935
Fernandez-Checa JC (2003) Redox regulation and signaling lipids in mitochondrial apoptosis. Biochem Biophys Res Commun 304:471–479
Conner EM, Grisham MB (1996) Inflammation, free radicals, and antioxidants. Nutrition 12:274–277
Vergely C, Maupoil V, Clermont G, Bril A, Rochette L (2003) Identification and quantification of free radicals during myocardial ischemia and reperfusion using electron paramagnetic resonance spectroscopy. Arch Biochem Biophys 420:209–216
Halliwell B (1989) Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol 70:737–757
Nixon RA, Cataldo AM (1994) Free radicals, proteolysis, and the degeneration of neurons in Alzheimer disease: how essential is the beta-amyloid link? Neurobiol Aging 15:463–469 (discussion 473)
Dreher D, Junod AF (1996) Role of oxygen free radicals in cancer development. Eur J Cancer 32A:30–38
Oberley LW (1988) Free radicals and diabetes. Free Radic Biol Med 5:113–124
Odetti P, Pesce C, Traverso N, Menini S, Maineri EP, Cosso L, Valentini S, Patriarca S, Cottalasso D, Marinari UM, Pronzato MA (2003) Comparative trial of N-acetyl-cysteine, taurine, and oxerutin on skin and kidney damage in long-term experimental diabetes. Diabetes 52:499–505
Wratten ML, Tetta C, Ursini F, Sevanian A (2000) Oxidant stress in hemodialysis: prevention and treatment strategies. Kidney Int Suppl 76:S126–S132
Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007) Trends in oxidative aging theories. Free Radic Biol Med 43:477–503
Miyata T, Taneda S, Kawai R, Ueda Y, Horiuchi S, Hara M, Maeda K, Monnier VM (1996) Identification of pentosidine as a native structure for advanced glycation end products in beta-2-microglobulin-containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc Natl Acad Sci USA 93:2353–2358
Kristal BS, Yu BP (1992) An emerging hypothesis: synergistic induction of aging by free radicals and Maillard reactions. J Gerontol 47:B107–B114
Davies KJ (1987) Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem 262:9895–9901
Tomasi A, Albano E, Banni S, Botti B, Corongiu F, Dessi MA, Iannone A, Vannini V, Dianzani MU (1987) Free-radical metabolism of carbon tetrachloride in rat liver mitochondria. A study of the mechanism of activation. Biochem J 246:313–317
Narkowicz CK, Vial JH, McCartney PW (1993) Hyperbaric oxygen therapy increases free radical levels in the blood of humans. Free Radic Res Commun 19:71–80
Duthie SJ, Gardner PT, Morrice PC, Wood SG, Pirie L, Bestwick CC, Milne L, Duthie GG (2005) DNA stability and lipid peroxidation in vitamin E-deficient rats in vivo and colon cells in vitro-modulation by the dietary anthocyanin, cyanidin-3-glycoside. Eur J Nutr 44:195–203
Anderson ME (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111–112:1–14
Witt EH, Reznick AZ, Viguie CA, Starke-Reed P, Packer L (1992) Exercise, oxidative damage and effects of antioxidant manipulation. J Nutr 122:766–773
Elia D, Stadler K, Horvath V, Jakus J (2006) Effect of soy- and whey protein-isolate supplemented diet on the redox parameters of trained mice. Eur J Nutr 45:259–266
Bounous G, Molson JH (2003) The antioxidant system. Anticancer Res 23:1411–1415
Sukkar SG, Cella F, Patriarca S, Furfaro AL, Abate F, Ferrari C, Balbis E, Traverso N, Cottalasso D (2008) Whey protein, as exclusively nitrogen source, controls food intake and promotes glutathione antioxidant protection in Sprague-Dawley rats. Mediterr J Nutr Metab 1:109–116
Balbis E, Patriarca S, Furfaro AL, Millanta S, Sukkar SG, Marinari UM, Pronzato MA, Cottalasso D, Traverso N (2009) Whey proteins influence hepatic glutathione after CCl4 intoxication. Toxicol Ind Health 25:325–328
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Traverso, N., Balbis, E., Sukkar, S.G. et al. Oxidative stress in the animal model: the possible protective role of milk serum protein. Mediterr J Nutr Metab 3, 173–178 (2010). https://doi.org/10.1007/s12349-010-0011-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12349-010-0011-1