Abstract
Introduction
In recent years, an increasing trend in the incidence of melanoma has been observed in Europe. Although early diagnosis and prompt intervention with local resection often results in positive outcomes, conversely, metastatic disease is still clinically challenging with a poor prognosis and a 5-year survival of around 30%. The growing awareness of melanoma biology and of antitumor immune responses has allowed the development of novel therapies targeted at specific molecular alterations occurring at advanced stages. This real-world analysis examined patients with melanoma in Italy, focusing on treatment patterns, outcome, time to discontinuation (TTD), and resource consumption.
Methods
Two retrospective observational analyses were conducted for BRAF+ patients with metastatic melanoma and those with a positive sentinel lymph node biopsy in an adjuvant setting, retrieving data from the administrative databases covering 13.3 million residents. The cohort melanoma BRAF+ in metastatic setting comprised 729 patients with targeted therapy (TT) (n = 671 with TT as first line and 79 as second line).
Results
Median TTD was 10.6 months in first line and 8.1 months in second line. Median overall survival from the start of first TT line was 27 months and was 11.8 months for patients with brain metastasis. In the dabrafenib plus trametinib patients, main healthcare resource consumption tended to increase in the presence of brain metastasis. The cohort with a positive sentinel lymph node biopsy under adjuvant therapy (n = 289) included 8% patients treated with dabrafenib plus trametinib or tested BRAF+, 5% BRAF wild-type, and 10% under immunotherapy.
Conclusion
Our findings provided an overview on TT utilization on metastatic melanoma patients in real clinical practice and highlighted an increased burden in brain metastatic patients.
Graphical Abstract

Similar content being viewed by others

Avoid common mistakes on your manuscript.
Why carry out this study? |
In recent decades, the number of melanoma have been constantly on the rise, especially in white people. While, with an early diagnosis and a prompt local resection, prognosis is favorable in most cases, metastatic melanoma can be a fatal disease and thus still represents an open clinical challenge. |
Mutations of the gene BRAF (v-raf murine sarcoma viral oncogene homolog B1) are frequently found in malignant melanoma and represent a therapeutic target for patients with advanced or metastatic stages. |
Little evidence is available in the Italian clinical practice context. This study was undertaken to provide an overview on the management of advanced melanoma in Italian real clinical practice, focusing on the role of novel targeted therapies in patients with BRAF-mutated metastatic disease and those with a positive sentinel lymph node biopsy in an adjuvant setting. |
What was learned from the study? |
Our data describe the journey of BRAF melanoma-mutant patients in Italian clinical practice, providing an overview of their therapeutic patterns and outcomes that could be of support for healthcare organizational management. |
Median overall survival and healthcare resource consumption revealed an increased burden in the presence of brain metastasis. |
Real-world data emerging from the present analysis were consistent and complemented the available literature on this topic, suggesting the use of such data could represent a useful tool to integrate the evidence generated by randomized controlled clinical trials. |
The results highlighted how brain metastases are associated with noticeably reduced survival, and also imply a relevant burden for both clinicians and the healthcare system, suggesting that this specific setting deserves further efforts in management optimization. |
Digital Features
This article is published with digital features, including a graphical abstract to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.23283902.
Introduction
Melanoma is one of the most aggressive skin cancers, although less common than other forms, and originates from the malignant transformation and uncontrolled proliferation of melanocytes [1]. Sun exposure has always been considered a causal factor for melanoma, along with genetic susceptibility. Intermittent and prolonged exposure seems to play a greater role than the age at which one is exposed to the sun, although exposure in childhood/adolescence results in a greater risk than at older ages [2, 3]. About 85% of cutaneous melanoma arising annually worldwide affect populations in North America, Europe, and Oceania, with the highest incidence in white people [4]. In recent years, an increasing trend in the incidence of melanoma has been reported for Europe which will likely continue in the next decades [5].
The Italian Cancer Registry Association (Associazione Italiana Registri Tumori) reported an incidence of around 12,700 new diagnoses of melanoma in Italy for year 2022 [6]. The age-standardized 5-year survival of patients with melanoma in Italy is 88% in males and 91% in females, higher than that of most other cancers [6]. The majority of new diagnoses are in the early stage with a positive prognosis after local resection [7]. However, in the setting of metastatic disease, the prognosis is very poor, with a 5-year survival of around 30% [8].
The most recent guidelines by an expert panel of the European Society for Medical Oncology included updated recommendations for the management of cutaneous melanoma, focusing on diagnosis, treatments, and follow-up [9]. Suspicious pigmented lesions are commonly evaluated using the so-called “ugly duckling” and the ‘ABCD’ rule: Asymmetry, Border irregularities, Color heterogeneity, Dynamics (intended as changes in color, elevation, or size) [9]. In suspected cases, the diagnostic path requires a full-thickness excisional biopsy with an adequate excised tissue margin followed by histology evaluation [9, 10].
Molecular characterization through mutational analysis for actionable mutations (the ones that can impact the clinical decisions) is mandatory in patients with resectable or unresectable disease (stages III–IV), and strongly recommended in high-risk resected melanoma (stage IIC), but not for earlier stages (I or IIA–IIB) [10].
In situ and pT1a melanoma are generally treated by a wide local excision with safety margins based on the tumor thickness. For later stages, further investigations are needed, given that the risk of sentinel lymph node metastases is significantly increased.
Over the last decade, a remarkable improvement in the management of advanced melanoma has been possible for the growing awareness of disease biology and the mechanisms of antitumor immune responses, which have allowed a more reliable approach to systemic therapies. In particular, a real revolution of the therapeutic landscape occurred after the introduction of immune checkpoint blockade with anti-CLTA-4 and anti-PD-1 antibodies, and the simultaneous results of selective inhibition of the MAP kinase pathway with BRAF and MEK inhibitors. These agents, initially used as monotherapies, and then also as combinations, provided significant clinical benefit at the unresectable stage III and metastatic stage IV patients. The following step was to assess their applicability as adjuvant therapy for high-risk resected stage III melanoma [11]. The BRAF-targeted agents currently approved by the Italian Medicine Agency (AIFA) for reimbursement by the National Health System (NHS) for unresectable metastatic melanoma are vemurafenib (VEM) alone or in association with cobimetinib (COB) since September 2016 [12], dabrafenib (DAB) alone or with trametinib (TRA) since January 2017 [13], and encorafenib (ENC) in association with binimetinib (BIN) since March 2020 [14]. In December 2019, dabrafenib plus trametinib were also approved as adjuvant therapies for BRAF+ patients with previously resected stage III melanoma with lymph node involvement [15]. In the same period, AIFA also released the new therapeutic indications and approved reimbursement of adjuvant treatment with pembrolizumab monotherapy [16] or nivolumab monotherapy [17], regardless of BRAF mutation status. To date, there is no approved treatment in Italy in the setting of stage II resected melanoma.
With the aim to obtain insights into the management of melanoma patients in clinical practice in Italy, we have performed a real-world analysis on the treatment patterns of patients with metastatic melanoma, with a focus on BRAF+ patients under target therapies (TT), exploited their outcomes in terms of overall survival, time to discontinuation and resource consumption. Furthermore, a description of patients with a positive sentinel lymph node biopsy and the treatment received has been provided.
Methods
Data Source
The data for the analysis were collected from the administrative, laboratory, and pathological anatomy databases of a sample of Italian Local Health Units (LHUs) across Italy, for a total coverage of around 13.3 million health-assisted subjects. Specifically, the following administrative databases were used: demographic database to get demographic information as age, sex, date of death; pharmaceuticals database for data on drugs reimbursed by the NHS as Anatomical-Therapeutic Chemical (ATC) code, number of units per package, number of packages dispensed, unit cost per package, prescription date; hospitalization database to collect discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), diagnosis-related group (DRG), and DRG-related charge; and the outpatient specialist service database reporting information about diagnostic tests and visits delivered. The data collected from administrative databases were linked with those retrieved from the pathological anatomy and laboratory databases, available in some LHUs, containing, respectively, the data concerning the histopathological examination and the laboratory test values.
In order to guarantee patient privacy, an anonymous univocal numeric code (Patient ID) was assigned to each beneficiary. The Patient ID permitted electronic linkage between the databases. No identifiers related to patients were provided. The anonymous code ensured the anonymity of the extracted data in full compliance with EU Data Privacy Regulation 2016/679 (GDPR) and Italian D.lgs. no. 196/2003, as amended by D.lgs. no. 101/2018. Furthermore, all the results have been produced exclusively in aggregate, so that it was not possible to identify the patients involved in the analysis from the extracted data, either directly or indirectly.
Patients’ informed consent was waived on the basis of the pronouncement of the Data Privacy Guarantor Authority (General Authorization for personal data treatment for scientific research purposes—no. 9/2014, December 11, 2014—published on the Official Gazette no. 301 on December 30, 2014), which states that data treatment is authorized without patient informed consent when the collection is impossible due to organizational reasons.
This observational study was performed in accordance with the principles of the Declaration of Helsinki. The project from which the analyses were drawn has been notified and approved by the local Ethics Committee of the LHUs involved in the study (Ethics Committee names, approval numbers, and dates are detailed in Supplementary Table S1).
Analysis Design
Two retrospective observational analyses were carried out on two separate cohorts: one focused on BRAF+ patients with melanoma in a metastatic setting, the other on patients with melanoma in an adjuvant setting with a positive sentinel lymph node biopsy. The two analyses are described separately in the following sections.
Cohort Melanoma BRAF Positive in Metastatic Setting
This cohort included all patients matching at least one of the following criteria: (1) at least a prescription for BRAF TT, namely dabrafenib + trametinib (DAB + TRA) (ATC codes L01XE23 + L01XE25) or vemurafenib + cobimetinb (VEM + COB) (ATC codes L01XE15 + L01XE38) or binimetinib + encorafenib (BIN + ENC) (ATC codes L01XE41 + L01XE46) from 1 January 2017 to 30 June 2021. From December 2019, treatment with DAB + TRA could also be prescribed to patients in adjuvant settings [15], hence patients with DAB + TRA were included from this date only if they did not have surgery or if starting such treatments after at least 16 weeks post-surgery; (2) at least one hospitalization with discharge diagnosis of melanoma (ICD-9-CM codes: 172.x) from 1 January 2010 to 30 June 2021 and evidence of metastasis (ICD-9-CM codes: 196–198) from 1 January 2010 to 30 June 2021 and at least one prescription for immunotherapy (IO) (ATC code: nivolumab: L01XC17, pembrolizumab: L01XC18) from 1 January 2017 to 30 June 2021 and tested BRAF-positive (from the pathological anatomy database) from 1 January 2010 to 30 June 2021. Among all included patients, the presence of radiotherapy (ICD-9-CM V58.0, procedural codes 92.2, 92.3) from the index date onwards was assessed.
Patients who tested negative for BRAF or with unknown mutational status were not included in the analysis. Exclusion criteria were applied based on the therapy encountered during the inclusion period, and a detailed explanation is provided in Supplementary Appendix S1. The number of treatment lines identified during the inclusion period was determined by looking through the whole data availability period within the databases (indicatively from 1 January 2010 to June 2021 at the latest). The therapies considered were TT, IO, and non-specific chemotherapy (CT, identified as the presence of ATC L01—excluding TT– or by DRG 410 or by procedure code 99.25). The first line with CT was considered only in the presence of evidence of melanoma diagnosis prior to the initiation of the line if no previous other cancers were identified and if it ended no more than 1 year before a subsequent line initiation. Patients were then stratified by first and second lines, and further subgrouped by type of therapeutic class prescribed (cohorts TT, IO, CT). The analyses were then focused on patients with TT. Characteristics collected at the time of first or second TT line initiation were age, sex, and presence within the database of brain metastases (ICD-9-CM code 198.3).
Furthermore, a focused analysis was conducted to estimate the mean daily dosage administered in patients with DAB + TRA, calculated in patients with at least two prescriptions, as total mg from the first to last prescription divided by the number of days between the first and last prescription. Healthcare resource consumption in patients with DAB + TRA with brain metastasis (in terms of number of prescriptions of TT, other drugs, hospitalizations, specialist visits, and diagnostic tests) was also evaluated.
Cohort with Adjuvant Therapy for Melanoma with a Positive Sentinel Lymph Node Biopsy
The patients included in this cohort had at least a record for positive biopsy of sentinel node (list codes reported in Supplementary Appendix S2), and surgical intervention (ICD-9-CM codes 86.2, 86.3, 86.4) from January 2019 to June 2021, who were grouped based on their records as follows: (1) subgroup BRAF+ : at least one prescription for DAB + TRA in patients within 16 weeks from surgery for patients treated starting from December 2019 OR tested positive for the BRAF test; (2) subgroup wild-type: BRAF tested AND BRAF negative; (3) subgroup BRAF unknown mutational status: patients with at least one prescription for IO (nivolumab and pembrolizumab) within 16 weeks from surgical intervention, starting from December 2019; and (4) subgroup patients not treated: patients without TT or IO. The index date was the date of treatment except for the untreated group, for whom the date of surgery was defined as index date. Patients previously treated with target or immune therapy were not considered. Lymph node dissection was evaluated by the presence of procedure codes 40.30, 40.40, 40.50.
Statistical Analysis
All the analyses were performed as descriptive. Means with standard deviations (SD) and medians were calculated for continuous variables, and for frequencies and percentages (%) for categorical variables. Kaplan–Meier curves were reported to evaluate the overall survival (OS) of TT patients in each line, defined as the time (in months) from therapy initiation to the date of death for any cause plus 1 day (OS was censored at the date of the end of database availability), and to evaluate the time to discontinuation (TTD) of each TT line, defined as time (in months) from therapy start to permanent discontinuation (plus last prescription duration for live patients, date of death plus 1 day for patients; TTD was censored at the date of the end of database availability). For patients in each line, the duration of treatment based on the period covered by the specific therapy prescribed have also been computed.
According to "Opinion 05/2014 on Anonymisation Techniques" drafted by the "European Commission Article 29 Working Party", the analyses involving ≤ 3 patients were not reported (NR), as potentially attributable to single individuals. All the statistical analyses were conducted using STATA SE, v.17.0.
Results
Cohort Melanoma BRAF+ in Metastatic Setting
A total of 873 patients with metastatic melanoma was identified by the presence of TT or IO therapies and melanoma discharge diagnosis. Among them, 83.5% (n = 729) were either treated with TT therapies or tested positive for BRAF+ and with IO: these were defined as BRAF+ and included in the study cohort (Fig. 1), while the remaining patients were not considered since their mutational status was unknown (n = 57) or tested BRAF negative (n = 87).
The first line of treatment is mostly represented by TT therapy (92.0%, n = 671, of which 577 with DAB + TRA, 10 with BIN + ENC, 84 with VEM + COB), followed by a small proportion of patients with IO (5.5%, n = 40) and with CT (2.5%, n = 18). Among metastatic melanoma BRAF+ patients in second line (n = 211), 37.4% (n = 79) received TT, 41.2% (n = 87) received IO and 21.4% (n = 45) were treated with CT. In the overall cohort melanoma BRAF positive in metastatic setting, 16.5% (n = 120) of patients had a radiotherapy after the inclusion in the analysis.
Treatment sequences are reported in Table 1: in the DAB + TRA group (n = 577, mean follow-up: 15.2 ± 12.9 months), 460 patients that did not move to a second line, 12.7% switched to IO, around 6.2% switched to CT and 1.4% to other TT. Pattern of treatment sequences for each calendar year is reported in Supplementary Table S2.
Almost all patients with BIN + ENC were still in first line at the end of the study: this result should be interpreted considering that those patients had a mean follow-up of 4.5 ± 2.7 months. Among the VEM + COB group (n = 87, mean follow-up: 26.4 ± 22.1 months) 19% switched to another TT as second line, followed by 14.3% and 8.3% that switched to IO and CT as second line, respectively. Most patients (66.7%) with CT in first line moved to DAB + TRA and 27.8% to other TT. Lastly, 86.8% patients with IO in first line switched to DAB + TRA. The different pattern of treatment sequences for each calendar year is reported in Supplementary Fig. 1.
As reported in Table 2, around 60% patients treated with TT were male, and the mean age at start of first-line treatment was 60 years (59.4 years in DAB + TRA, 59.8 years in BIN + ENC, 63.8 years in VEM + COB). Of the 79 patients treated with TT as second line (62% male), overall mean age was 62 years: 60.9 years among BIN + ENC, 62.9 years among DAB + TRA, 57.1 years VEM + COB patients. Considering patients with at least a metastasis reported in the administrative databases (n = 323 in first line and 44 in second line), brain metastases were detected in 37.8% of TT patients in first line, specifically 35.5% in DAB + TRA- and 51% in VEM + COB-treated patients and in 47.7% of patients in second line.
Patients with TT in first line were followed-up for a median time of 11.3 months, during which 37% of patients died, and this proportion reached 74.6% among patients with brain metastasis (n = 122). During a median follow-up of 9.7 months, 44.3% of patients in second TT line died. Median (95% CI) overall survival from the start of first TT line was of 27 (23.2–31.1) months (Fig. 2a), and of 11.8 (10.0–16.0 months) for patients with brain metastasis (Fig. 2b).
Regarding patients with TT as second line, median survival was of 13.9 months (Fig. 3). Due to the low sample size, the overall survival of patients in second line with brain metastasis could not be calculated. Median [95% CI] time to discontinuation was of 10.6 [9.5–12.0] months for first line (5.7 [median not reached] months for BIN + ENC, 11.0 [9.6–12.1] months for DAB + TRA and 9.2 [6.3–11.5] months for VEM + COB), with a proportion of discontinuing patients of 62% overall. A similar percentage was observed for all TT patients that discontinued the second line (59.5%), with a median time of 8.1 [5.8–11.0] months (6.0 [2.9-upper limit not reached] months for BIN + ENC, 8.2 [5.3–11.4] months for DAB + TRA and 5.0 [1.0-upper limit not reached] months for VEM + COB).
A focused analysis was performed on DAB + TRA patients (Table 3) who were followed-up for a median time of 11.1 (patients in first line, n = 577) and 9.8 months (patients in second line, n = 59). Median duration of treatment (as period covered by treatment prescriptions) was 7.3 for all DAB + TRA patients (7.3 months for first line and 6.4 for second line). Mean daily dosage (Table 3) was 286.1 mg (DAB) + 1.4 mg (TRA) (the posology indicated to datasheets reported a total of 300 mg DAB and 2 mg TRA when administered together). Mean daily dosage in patients in first and second line was 287.3 mg (DAB) + 1.4 mg (TRA) and 278.6 mg (DAB) + 1.4 mg (TRA), respectively. A slightly high dosage was observed in patients with brain metastasis: 288.8 + 1.4 mg. Ultimately, the healthcare resource consumption evaluated for DAB + TRA patients while on treatment comprised a mean number of 9.2 prescriptions for TT, 6.0 prescriptions for other drugs, 6.9 and 10.5 mean number of visits and tests, respectively, and 0.3 hospitalizations. Focusing on the subpopulation of patients with brain metastasis treated with DAB + TRA, each patient had a mean number of 10.5 prescriptions for TT, 7.9 prescriptions for other drugs, 11.1 and 13.6 mean number of visits and tests, respectively, and 0.9 hospitalizations during treatment period (Fig. 4; Table 4).
Cohort with Adjuvant Therapy for Melanoma with a Positive Sentinel Lymph Node Biopsy
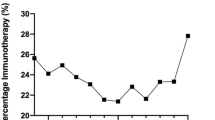
Among adjuvant melanoma patients included (n = 289): 8% (n = 23) were treated with DAB + TRA or tested BRAF positive (BRAF +), 5% (n = 14) were deemed as BRAF negative (BRAF WT), 10% (n = 28) was treated with IO and not tested for BRAF (BRAF unknown), and 77% (n = 224) not treated and not tested for BRAF (Fig. 5). All patients BRAF+ had at least a prescription for TT treatment by considering the all data available period. Mean age of included patients was 63.3 years, 58.8% were male and 43.9% had a lymph node dissection. In order to investigate whether the COVID pandemic had an impact on the management of patients with adjuvant therapy, the number of patients per quarter in each inclusion year has been reported in Fig. 6; no clear pattern was observed.
Discussion
This analysis investigated a representative sample of patients with metastatic melanoma in a real-life clinical setting in Italy, with the goal of describing the current state-of-art on their therapeutic management, and the clinical and public healthcare rebounds. In particular, a special attention was given to the carriers of BRAF gene mutations, and the use of BRAF target therapies on patients' survival, duration of treatment, and the related burden on healthcare resource consumption.
The demographics of our included patients were largely consistent with other previously published international and national reports, with a slightly predominance of male gender and an average age at the initiation of first line therapy of around 60 years [18, 19].
The treatment sequences used in our sample mirror the general attitude by oncologists and the literature data of the last decade. It is well established that both TT and IO have revolutionized the perspectives of BRAF-positive metastatic melanoma, with further positive expectations emerging from the synergic effects of combined immune-checkpoint inhibitors and targeted agents [20, 21].
A small proportion of patients (16.5%) received radiotherapy; the combination of TT or IO with radiotherapy (as pre-induction or post-escape) was demonstrated to be a promising therapeutic option either in metastatic or no-metastatic settings; however, to date, still more evidence is needed to confirm these results [22].
In oncology, several endpoints have been used to evaluate the clinical benefit of anticancer therapies: among them, overall survival is commonly considered as the gold standard in randomized controlled trials (RCTs), but this implies broader follow-ups and large patient sample size [23]. On the other hand, in real-world evidence studies based on unselected larger populations of patients, TTD has been suggested to be a more reliable proxy clinical endpoint [24, 25]. In our analysis, we found a great proportion of patients who discontinued TT therapy, specifically 62% in first line and 59.5% in second line, regardless of the therapeutic combinations. Moreover, the median time to discontinuation ranged from 9.2 to 11 months and from 5 to 8.1 in first and second lines, respectively. When looking at specific BRAF therapies, the median time to discontinuation of DAB + TRA (11 months) in first line was consistent with that reported by Aglietta et al. [19] (10.3 months), in which disease progression was addressed as the main reason for discontinuation. Indeed, there might be several reasons for therapy discontinuation in patients with a BRAF-mutated metastatic melanoma, mainly disease progression or toxicity. Evidence has shown that, although resistance or toxicity to TT can occur, many patients maintain long-term disease control, confirming that the advent of BRAF/MEK inhibitors has greatly improved the management of patients with BRAF-mutated metastatic melanoma [26].
The median OS after initiating first-line TT was 27 months. In second line, the median OS decreased to 13.9 months in the overall population. These findings do not differ substantially from those of the DESCRIBE II study, a retrospective chart review of patients with BRAF V600-mutated unresectable stage III/IV melanoma receiving the DAF + TRA combination. Among 271 patients (92.6% with stage IV melanoma, and 36.5% with brain metastases) analyzed for treatment patterns and duration, clinical outcomes, and tolerability, those who were BRAF-naïve and received DAB + TRA, the median OS was 20.0 months in first line and 15.1 months in second line [27]. The slight discrepancy might be explicated by the different therapy purpose of the DESCRIBE II study, which investigated DAB + TRA therapy in compassionate use settings from various healthcare entities of six participating countries (Italy, Australia, the Netherlands, Lithuania, Spain, and Czech Republic), while our analysis comprised all TT therapies, and using a different study design.
Our findings corroborate the view that brain metastases are the most frequent type of metastases and the worst prognostic factors in these patients, as they directly cause death in 60–70% of melanoma patients [28]. We noticed their occurrence among patients with metastasis detected within the database to be 38% in all TT patients in first line, and around 48% of those in second line. Moreover, the median overall survival since the start of TT as first-line therapy was strikingly lower in patients with brain metastases than in those without, 11.8 and 27 months, respectively. Median OS for metastatic melanoma patients treated with TT has been reported in the literature to span between 18 and 33 months from initiation of TT [29, 30].
Our data revealed that, in both first and second line, the average daily dosage of the DAB + TRA combination administered to our patients was adequately in line with the posology indicated in the datasheets, with only a slightly reduction for TRA (1.4 mg in the present analysis compared to 2 mg recommended dosage). As expected, a slightly increased dosage of DAB, but not TRA, was noticed in patients with brain metastasis These data are consistent with a phase 1 dose-escalation trial of DAB in ten patients [31].
The subpopulation of patients with brain metastasis receiving DAB + TRA combination was also evaluated for healthcare resource consumption during the period of treatment. Compared to the overall DAB + TRA patients, we observed a trend of increased numbers for visits and tests, followed by prescriptions for TT and other drugs, and hospitalizations. Recently, a single-center study in a Dutch hospital investigated healthcare consumption only in the 3-month period prior to death in this specific subgroup, revealing that about 64% patients were visited inj the emergency room for neurological or gastric symptoms, but only a small proportion of visits were likely related to antitumor treatments [32]. The increased healthcare resource consumption reported here could be contextualized with another large study conducted on 6076 US patients which compared brain metastasis-related resource use and healthcare costs in the 6-month period before metastasis detection and in the 12-month period after. The results showed significant post- versus pre-period differences in both healthcare use and related costs, mostly driven by inpatient and outpatient services, similar to our findings. Even though in the present analysis we did not carry out an evaluation over time of the possible incremental healthcare consumption in the setting of melanoma patients with brain metastasis receiving DAB + TRA, taken together these data underline the elevated burden associated with brain metastases and possible room for improvement in the management of this specific population [33].
In the adjuvant cohort, we found a consistent proportion of untreated patients, and this percentage appears to be higher compared to other previous studies. Recently, a multicenter German study reported real-world data of 904 BRAF mutant patients from 13 skin cancer centers and investigated the frequencies and the reasons underlying treatment decisions (adjuvant therapy yes/no). The authors found that, in the various participating centers, the proportion of the those who opted for a systemic adjuvant treatment ranged between 60% and 97%, and that the most common reasons against adjuvant treatment were older age, fear of adverse events, and impaired quality of life, more markedly seen in females [34]. The differences observed in our study could be explained by the methodology applied, since, for inclusion in the adjuvant cohort, we did not consider patients previously treated with BRAF therapy or with IO, or by data source, because patients enrolled in clinical trials are not retrievable from administrative databases. Therefore, we may have overestimated the proportion of untreated patients. It should also be taken into account that adjuvant treatments like DAB + TRA, nivolumab, and pembrolizumab were approved for reimbursement in Italy in December 2019, thus those who received these therapies before that time were feasibly off label or in compassionate use.
In the adjuvant population stratified by years of observation, we did not notice any substantial change over time in the number of patients per quarter for each inclusion year. This point deserves special attention, as the COVID-19 pandemic has been the central global emergency during the past 2 years, resulting in large-scale adaptations in healthcare provisions. A recent meta-analysis by Seretis et al. [35] investigated the relationship of the pandemic outbreak and lockdowns with melanoma diagnoses and related burdens across Europe. Through an extensive literature search, the authors selected 25 studies involving 32,231 patients and found significantly increased mean Breslow thickness, ulceration rates, and resultant tumor staging in the post-COVID era, indicating that the pandemic caused a heavier tumor burden and faster disease progression state due to healthcare adaptations, especially during the earlier pandemic waves. The discrepancy with our data can be explained by the fact that sentinel node biopsy may not be an indicator of a first melanoma diagnosis, and that maybe patients at risk were already checked and managed in the pre-COVID period.
Our data should be interpreted considering the following limitations due to the retrospective observational nature of the study and the data source used. The first limitation concerns the data included in the pathological anatomy database, which contains different types of information that are often reported as text. Thus, data extraction is not straightforward, and it may require a level of visual inspection that is not possible when collecting a large amount of data. In addition, a high degree of variability can be found between different databases. Regarding administrative databases, the main limitation is represented by the lack of primary care data and clinical data, particularly related to patients’ molecular and mutational profiles, status of melanoma, and other undetectable confounders that could have affected the results. Furthermore, we could not retrieve the cause of death, nor the reasons behind the choice of therapy or the discontinuation. The difference observed among follow-up duration of each TT reflected the different timing of the reimbursability by the NHS. Ultimately, patients entering clinical trials are not captured in the administrative databases, therefore the analysis of treatment sequences (metastatic cohort) and the proportion of untreated patients (adjuvant) could be underestimated.
Conclusions
Investigations based on real-world data can gain insights into the understanding of the management of oncology patients in clinical practice to better guide treatment decisions in the routine care in wide unselected populations of patients [36]. Our study fits in this context by providing a general overview regarding the utilization of TT on patients with melanoma in settings of clinical practice. The characteristics of patients were consistent with those reported in the literature, as well as the outcome analyses in terms of overall survival and time to discontinuation, thus indicating that the generation of evidence captured in real-world settings could be a useful tool to provide data that could complement and integrate with those generated by randomized controlled clinical trials [37, 38]. Furthermore, the clinical care of patients was also investigated in terms of their access to healthcare resources, which highlighted an increased burden in brain metastatic patients, suggesting the optimization of the management of this specific population.
References
Lopes J, Rodrigues CMP, Gaspar MM, Reis CP. Melanoma management: from epidemiology to treatment and latest advances. Cancers (Basel). 2022;14(19):4652. https://doi.org/10.3390/cancers14194652.
Correya T, Duncan Z, Garcia N, Amu-Nnadi C, Broman K. Incidence and risk factors for incidental cancer on melanoma wide excisions. J Surg Res. 2023;284:24–8. https://doi.org/10.1016/j.jss.2022.11.045.
Gandini S, Sera F, Cattaruzza MS, Pasquini P, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41(1):45–60. https://doi.org/10.1016/j.ejca.2004.10.016.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. https://doi.org/10.1002/ijc.25516.
Crispo A, Corradin MT, Giulioni E, et al. Real life clinical management and survival in advanced cutaneous melanoma: The Italian Clinical National Melanoma Registry experience. Front Oncol. 2021;11: 672797. https://doi.org/10.3389/fonc.2021.67279.
Gruppo di Lavoro AIOM—AIRTUM—Fondazione AIOM I NUMERI DEL CANCRO IN ITALIA 2022. https://www.aiom.it/wp-content/uploads/2022/12/2022_AIOM_NDC-web.pdf. Accessed 24 April 2023.
Mohr P, Ascierto P, Arance A, et al. Real-world treatment patterns and outcomes among metastatic cutaneous melanoma patients treated with ipilimumab. J Eur Acad Dermatol Venereol. 2018;32(6):962–71. https://doi.org/10.1111/jdv.14633.
Switzer B, Puzanov I, Skitzki JJ, Hamad L, Ernstoff MS. Managing metastatic melanoma in 2022: a clinical review. JCO Oncol Pract. 2022;18(5):335–51. https://doi.org/10.1200/OP.21.00686.
Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U, ESMO Guidelines Committee. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1884–901. https://doi.org/10.1093/annonc/mdz411.
AIOM Linee Guida Melanoma. Edizione 2021. https://www.aiom.it/linee-guida-aiom-2021-melanoma/. Last accessed on 24 April 2023.
Testori AAE, Chiellino S, van Akkooi ACJ. Adjuvant therapy for melanoma: past, current, and future developments. Cancers (Basel). 2020;12(7):1994. https://doi.org/10.3390/cancers12071994.
Gazzetta Ufficiale—Regime di rimborsabilità e prezzo di vendita del medicinale per uso umano «Cotellic (cobimetinib)». (Determina n. 1204/2016). https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2016-10-01&atto.codiceRedazionale=16A07019. Last accessed on 24 April 2023.
Gazzetta Ufficiale Mekinist—Classificazione del medicinale per uso umano «Mekinist» (Determina n. 1546/2016). https://www.gazzettaufficiale.it/eli/id/2017/01/03/16A09047/sg. Last accessed on 24 April 2023.
Gazzetta Ufficiale—Riclassificazione del medicinale per uso umano «Braftovi» (Determina n. 289/2020). https://www.gazzettaufficiale.it/eli/id/2020/04/09/20A01991/sg. Last accessed on 24 April 2023.
Gazzetta Ufficiale—Regime di rimborsabilità e prezzo a seguito di nuove indicazioni terapeutiche del medicinale per uso umano «Mekinist». (Determina n. 1795/2019). https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2019-12-16&atto.codiceRedazionale=19A07822&elenco30giorni=false. Last Accessed on 24 April 2023.
Gazzetta Ufficiale—Regime di rimborsabilita' e prezzo a seguito di nuove indicazioni terapeutiche del medicinale per uso umano «Keytruda». (Determina n. 1762/2019). https://www.gazzettaufficiale.it/eli/id/2019/12/10/19A07733/sg. Last accessed on 24 April 2023.
Gazzetta Ufficiale—Regime di rimborsabilita' e prezzo, a seguito di nuove indicazioni terapeutiche, del medicinale per uso umano «Opdivo». (Determina n. 1799/2019). https://www.gazzettaufficiale.it/eli/id/2019/12/17/19A07828/SG. Last accessed on 24 April 2023.
Haferkamp S, Alter M, Debus D, et al. Patients with BRAF-mutant advanced/metastatic melanoma: original research on the treatment reality in Germany and Austria in the era of choice. Adv Ther. 2020;37(8):3619–29. https://doi.org/10.1007/s12325-020-01430-x.
Aglietta M, Chiarion-Sileni V, Fava P, et al. Retrospective chart review of dabrafenib plus trametinib in patients with metastatic BRAF V600-mutant melanoma treated in the individual patient program (DESCRIBE Italy). Target Oncol. 2021;16(6):789–99. https://doi.org/10.1007/s11523-021-00850-1.
Giugliano F, Crimini E, Tarantino P, et al. First line treatment of BRAF mutated advanced melanoma: Does one size fit all? Cancer Treat Rev. 2021;99: 102253. https://doi.org/10.1016/j.ctrv.2021.102253.
Pires da Silva I, Zakria D, Ahmed T, et al. Efficacy and safety of anti-PD1 monotherapy or in combination with ipilimumab after BRAF/MEK inhibitors in patients with BRAF mutant metastatic melanoma. J Immunother Cancer. 2022;10(7):e004610. https://doi.org/10.1136/jitc-2022-004610.
Tagliaferri L, Lancellotta V, Fionda B, et al. Immunotherapy and radiotherapy in melanoma: a multidisciplinary comprehensive review. Hum Vaccine Immunother. 2022;18(3):1903827. https://doi.org/10.1080/21645515.2021.1903827.
Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13(Suppl 2):19–21. https://doi.org/10.1634/theoncologist.13-S2-19.
Blumenthal GM, Gong Y, Kehl K, et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann Oncol. 2019;30(5):830–8. https://doi.org/10.1093/annonc/mdz060.
Rivera DR, Henk HJ, Garrett-Mayer E, et al. The Friends of Cancer Research Real-World Data Collaboration Pilot 2.0: methodological recommendations from oncology case studies. Clin Pharmacol Ther. 2022;111(1):283–92. https://doi.org/10.1002/cpt.2453.
Stege H, Haist M, Schultheis M, et al. Discontinuation of BRAF/MEK-directed targeted therapy after complete remission of metastatic melanoma—a retrospective multicenter ADOReg study. Cancers (Basel). 2021;13(10):2312. https://doi.org/10.3390/cancers13102312.
Atkinson V, Sandhu S, Hospers G, et al. Dabrafenib plus trametinib is effective in the treatment of BRAF V600-mutated metastatic melanoma patients: analysis of patients from the dabrafenib plus trametinib Named Patient Program (DESCRIBE II). Melanoma Res. 2020;30(3):261–7. https://doi.org/10.1097/CMR.0000000000000654.
Gutzmer R, Vordermark D, Hassel JC, et al. Melanoma brain metastases—interdisciplinary management recommendations 2020. Cancer Treat Rev. 2020;89: 102083. https://doi.org/10.1016/j.ctrv.2020.102083.
Isoardo A, Ferrero M, Grande E, Fruttero C. 1ISG-010 Real-world evidence of high-cost drugs for metastatic melanoma: effectiveness, compliance to clinical practice guidelines and economic evaluation. In: Proceedings of the section 1: introductory statements and governance. British Medical Journal Publishing Group, 2019; p. A5.1–A5.
Luke JJ, Ghate SR, Kish J, et al. Targeted agents or immuno-oncology therapies as first-line therapy for BRAF-mutated metastatic melanoma: a real-world study. Future Oncol. 2019;15(25):2933–42. https://doi.org/10.2217/fon-2018-0964.
Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901. https://doi.org/10.1016/S0140-6736(12)60398-5.
Eggen AC, Hospers GAP, Bosma I, Kramer MCA, Reyners AKL, Jalving M. Anti-tumor treatment and healthcare consumption near death in the era of novel treatment options for patients with melanoma brain metastases. BMC Cancer. 2022;22(1):247. https://doi.org/10.1186/s12885-022-09316-7.
VekemanF, Cloutier M, Yermakov S, et al. incremental cost of brain metastases among patients with metastatic melanoma. Value Health 2013;16:138. https://doi.org/10.1016/j.jval.2013.03.674.
Lodde G, Forschner A, Hassel J, et al. Factors influencing the adjuvant therapy decision: results of a real-world multicenter data analysis of 904 melanoma patients. Cancers (Basel). 2021;13(10):2319. https://doi.org/10.3390/cancers13102319.
Seretis K, Bounas N, Gaitanis G, Bassukas I. A meta-analysis on the impact of the COVID-19 pandemic on cutaneous melanoma diagnosis in Europe. Cancers (Basel). 2022;14(24):6085. https://doi.org/10.3390/cancers14246085.
Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–7. https://doi.org/10.1056/NEJMsb1609216.
Di Maio M, Perrone F, Conte P. Real-world evidence in oncology: opportunities and limitations. Oncologist. 2020;25(5):e746–52. https://doi.org/10.1634/theoncologist.2019-0647.
Karim S, Booth CM. Effectiveness in the absence of efficacy: cautionary tales from real-world evidence. J Clin Oncol. 2019;37(13):1047–50. https://doi.org/10.1200/JCO.18.02105.
Acknowledgements
Funding
Novartis Farma S.p.A. purchased the study report that is the basis for this manuscript. The journal’s Rapid Service and Open Access was funded by Novartis Farma S.p.A. This manuscript was developed with Novartis Farma S.p.A and CliCon S.r.l. Società Benefit. The agreement signed by Clicon S.r.l. and Novartis Farma S.p.A does not create any entity, joint venture or any similar relationship between parties. Clicon S.r.l. is an independent company. Neither CliCon S.r.l. nor any of their representatives are employees of Novartis Farma S.p.A for any purpose.
Authorship
All the listed coauthors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take full responsibility for the integrity of the work, and have approved this version to be published.
Author Contributions
Conceptualization, Ilaria Gioia Marcon, Diletta Valsecchi, Lorenza Durso, Laura Catena and Luca Degli Esposti; Data curation, Luca Degli Esposti; Investigation, Luca Degli Esposti; Methodology, Diletta Valsecchi and Diego Sangiorgi; Supervision, Luca Degli Esposti; Validation, Eleonora Premoli, Laura Catena and Luca Degli Esposti; Visualization, Ilaria Gioia Marcon, Diletta Valsecchi, Eleonora Premoli and Valentina Perrone; Writing—original draft, Valentina Perrone; Writing—review and editing, Ilaria Gioia Marcon, Diletta Valsecchi, Lorenza Durso, Eleonora Premoli, Diego Sangiorgi, Valentina Perrone, Laura Catena and Luca Degli Esposti. All authors have read and agreed to the published version of the manuscript.
Disclosures
Ilaria Gioia Marcon, Diletta Valsecchi, Lorenza Durso, Eleonora Premoli, Laura Catena are employees of Novartis Farma S.p.A., Italy. Valentina Perrone and Luca Degli Esposti L. are employees of CliCon S.r.l. Società Benefit, Italy. Diego Sangiorgi was employee of CliCon S.r.l. Società Benefit, Italy at the time the manuscript was developed, and it is currently employed by Maria Cecilia hospital, gvm care & research.
Compliance with Ethics Guidelines
This observational study was performed in accordance with the principles of the Declaration of Helsinki. The project from which the analyses were drawn has been notified and approved by the local Ethics Committee of the LHUs involved in the study (Ethics Committee names, approval numbers and dates are detailed in Supplementary Table S1).
Data Availability
All data used for the current study are available upon reasonable request next to CliCon s.r.l. which is the body entitled of data treatment and analysis by Local Health Units.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Marcon, I.G., Valsecchi, D., Durso, L. et al. Real-World Evaluation of the Management, Treatment Pathways and Outcome of Melanoma Patients with Target Therapies in Italy. Adv Ther 40, 3875–3895 (2023). https://doi.org/10.1007/s12325-023-02578-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02578-y