Abstract
Introduction
Cetuximab plus FOLFIRI (leucovorin, fluorouracil, and irinotecan) is the preferred first-line therapy for RAS and BRAF wild-type (RBWT) metastatic colorectal cancer (mCRC). To counter chemotherapy-induced side effects, use of maintenance therapy is suggested. Therefore, we evaluated the efficacy and safety of cetuximab maintenance therapy in patients after effective completion of first-line induction therapy.
Methods
This prospective study enrolled untreated patients with mCRC RBWT who received first-line cetuximab plus FOLFIRI therapy. Following this, patients with treatment response either entered observation (stop treatment) or maintenance treatment 1 (cetuximab plus irinotecan) groups. After 6–12 cycles of maintenance treatment 1, patients entered maintenance treatment 2 (cetuximab only). If a patient progressed on maintenance 2, cetuximab plus FOLFIRI was reintroduced. The primary end point was failure-free survival (FFS), whereas the secondary end points included disease control rate (DCR), objective remission rate (ORR), and progression-free survival (PFS). Safety events were also evaluated.
Results
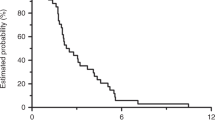
Among 79 enrolled patients, 72 completed first-line treatment effectively (DCR 91.1%, ORR 63.9%) and 44 entered maintenance 1 [median PFS 1 (mPFS, maintenance 1) 6.1 months, 95% confidence interval (CI) 6.0–6.2; DCR 56.8%; ORR 22.7%]. Of them, 21 entered maintenance treatment 2 (mPFS2 8.7 months, 95% CI 3.3–14.1; DCR 28.6%; ORR 4.8%). Median FFS (mFFS) was significantly longer in the maintenance 1 group compared with the observation group [12.7 vs. 3.0 months; hazard ratio (HR) 0.202, 95% CI 0.111–0.369; P < 0.001]. Overall, mFFS was 19.0 and 9.3 months in maintenance and observation groups, respectively (HR 0.211, 95% CI 0.117–0.380; P < 0.001). Rash acneiform, mucositis, and asthenia were commonly observed adverse events during maintenance treatment.
Conclusion
Maintenance treatment with cetuximab after first-line therapy significantly improved FFS, with an acceptable safety profile in untreated patients with mCRC RBWT.
Trial Registration
Retrospectively registered, 2019/10/02, Chinese Clinical Trial Registry, ChiCTR number 1900026360.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Chen W-Q, Zheng R-S, Zhang S-W, Zeng H-M, Zou X-N. The incidences and mortalities of major cancers in China, 2010. Chin J Cancer. 2014;33:402–5.
Zhang L, Cao F, Zhang G, et al. Trends in and predictions of colorectal cancer incidence and mortality in China From 1990 to 2025. Front Oncol. 2019;9:98.
Oonnell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5.
Van Cutsem E, Oliveira J, ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):61–3.
Benson AB, Venook AP, Al-Hawary MM, et al. Anal carcinoma, version 2. 2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2018;16:852–71.
Van Cutsem E, Köhne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–8.
Bokemeyer C, Kohne C-H, Ciardiello F, et al. Treatment outcome according to tumor RAS mutation status in OPUS study patients with metastatic colorectal cancer (mCRC) randomized to FOLFOX4 with/without cetuximab. J Clin Oncol. 2014;32:3505–3505.
Tabernero J, Van Cutsem E, Díaz-Rubio E, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25:5225–322.
Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8.
Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–9.
Santini D, Vincenzi B, Addeo R, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol. 2012;23:2313–8.
Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426–34.
Dueland S, Guren TK, Hagness M, et al. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg. 2015;261:956–60.
Berry SR, Cosby R, Asmis T, et al. Continuous versus intermittent chemotherapy strategies in metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2015;26:477–85.
Rossi L, Vakiarou F, Zoratto F, et al. Factors influencing choice of chemotherapy in metastatic colorectal cancer (mCRC). Cancer Manag Res. 2013;5:377–85.
Simkens LHJ, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385:1843–52.
Hanna DL, Lenz H-J. Novel therapeutics in metastatic colorectal cancer: molecular insights and pharmacogenomic implications. Expert Rev Clin Pharmacol. 2016;9:1091–108.
Goey KKH, Elias SG, van Tinteren H, et al. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol. 2017;28:2128–34.
Chibaudel B, Tournigand C, Bonnetain F, et al. Therapeutic strategy in unresectable metastatic colorectal cancer: an updated review. Ther Adv Med Oncol. 2015;7:153–69.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–8.
Gligorov J, Doval D, Bines J, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1351–60.
Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96.
Drugs@FDA: FDA approved drug products: cetuximab. 2004. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. Accessed 4 May 2019.
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65.
Van Cutsem E, Lenz H-J, Köhne C-H, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700.
Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47.
Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2016;3:194–201.
Ciardiello F, Lenz H, Peeters M, et al. Right or left metastatic colorectal cancer: will the side change your treatment? Copenhagen, Denmark; 10 October 11:15–12:50. https://www.esmo.org/Conferences/ESMO-2016-Congress/Webcasts. Accessed 4 May 2019.
Shapiro JD, Thavaneswaran S, Underhill CR, et al. Cetuximab alone or with irinotecan for resistant KRAS-, NRAS-, BRAF- and PIK3CA-wild-type metastatic colorectal cancer: the AGITG randomized phase II ICECREAM study. Clin Colorectal Cancer. 2018;17:313–9.
Wasan H, Meade AM, Adams R, et al. Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol. 2014;15:631–9.
Ciardiello F, Normanno N, Martinelli E, Troiani T. Cetuximab beyond progression in RAS wild type (WT) metastatic colorectal cancer (mCRC): the CAPRI-GOIM randomized phase II study of FOLFOX versus FOLFOX plus cetuximab. Ann Oncol. 2015;26(suppl 4):120–1.
Feng Q, Wei Y, Ren L, et al. Efficacy of continued cetuximab for unresectable metastatic colorectal cancer after disease progression during first-line cetuximab-based chemotherapy: a retrospective cohort study. Oncotarget. 2016;7:11380–96.
Fora AA, McMahon JA, Wilding G, et al. A phase II study of high-dose cetuximab plus irinotecan in colorectal cancer patients with KRAS wild-type tumors who progressed after standard dose of cetuximab plus irinotecan. Oncology. 2013;84:210–3.
Xu R, Li Y, Luo H, et al. Continuing single-agent capecitabin as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2015;33:3580–3580.
Guren TK, Thomsen M, Kure EH, et al. Cetuximab in treatment of metastatic colorectal cancer: final survival analyses and extended RAS data from the NORDIC-VII study. Br J Cancer. 2017;116:1271–8.
Aranda E, García-Alfonso P, Benavides M, et al. First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: phase II randomised MACRO2 TTD study. Eur J Cancer. 2018;101:263–72.
Petrioli R, Francini E, Cherri S, et al. Capecitabine plus oxaliplatin and bevacizumab, followed by maintenance treatment with capecitabine and bevacizumab for patients aged > 75 years with metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17:e663–e66969.
Yalcin S, Uslu R, Dane F, et al. Bevacizumab + capecitabine as maintenance therapy after initial bevacizumab + XELOX treatment in previously untreated patients with metastatic colorectal cancer: phase III ‘Stop and Go’ study–results a Turkish Oncology Group trial. Oncology. 2013;85:328–35.
Pinto C, Barone CA, Girolomoni G, et al. Management of skin toxicity associated with cetuximab treatment in combination with chemotherapy or radiotherapy. Oncologist. 2011;16:228–38.
Van Cutsem E, Tejpar S, Vanbeckevoort D, et al. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST study. J Clin Oncol. 2012;30:2861–8.
Acknowledgements
We thank all the participants of this study.
Funding
The study was funded by the Joint Funds for the innovation of science and technology Fujian province (2018Y9031, 2018Y9032) and Fujian Medical University Qihang Foundation (2018QH1035). Sponsors of the study also funded the journal’s Rapid service fee.
Medical Writing Assistance
Medical writing support under authors’ direction was provided by Priyanka Bannikoppa, PhD, and Anuradha Nalli, PhD (Indegene Pvt. Ltd., Bangalore), as funded by the author.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
The abstract was submitted to ASCO, 2019 held in Chicago from May 31–June 4.
Disclosures
Tao Jing, Hao Chen, Xiaoyan Lin, Jianwei Zheng, Bin Du, Baoyu Yang, Qing Liu, Dongta Zhong, Xinli Wang, Han Wang, Mengxin Lin, Jinhuo Lai and Peifeng Hou have nothing to disclose.
Compliance with Ethics Guidelines
This study was conducted in accordance with the Declaration of Helsinki, 1964, as revised in 2013 and the International Council for Harmonization guideline E6: Good Clinical Practice (GCP). The study was approved by the Institutional Review Board (IRB number: 2015KY033) of Fujian Medical University Union Hospital and the investigators obtained informed consent from each patient. The study protocol was registered at Chinese Clinical Trial Registry, ChiCTR number 1900026360.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12162018.
Rights and permissions
About this article
Cite this article
Jiang, T., Chen, H., Zheng, J. et al. Cetuximab Maintenance Therapy in Patients with Unresectable Wild-Type RAS and BRAF Metastatic Colorectal Cancer: A Single-Institute Prospective Study. Adv Ther 37, 2829–2840 (2020). https://doi.org/10.1007/s12325-020-01360-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01360-8