Abstract
Introduction
The phase III MPACT trial in patients with metastatic pancreatic cancer (MPC) demonstrated superior efficacy of nab-paclitaxel (nab-P) plus gemcitabine (Gem) compared with Gem monotherapy, including the primary endpoint of overall survival (OS; median 8.7 vs. 6.6 months; hazard ratio [HR] 0.72; P < 0.001). A significant treatment difference favoring nab-P + Gem over Gem was observed for OS in patients treated in North America. The majority of patients were from the US (88%) with only 12% from Canada. Healthcare systems and treatment patterns are different between the 2 countries, and there is limited published information on outcomes of Canadian patients treated with first-line nab-P + Gem. This analysis evaluated efficacy and safety outcomes in Canadian patients in the MPACT trial.
Methods
Treatment-naive patients with MPC (N = 861) received either nab-P 125 mg/m2 + Gem 1000 mg/m2 on days 1, 8, and 15 every 4 weeks or Gem 1000 mg/m2 weekly for the first 7 of 8 weeks (cycle 1) and then on days 1, 8, and 15 every 4 weeks (cycle ≥2).
Results
The MPACT trial enrolled 63 patients in Canada. Baseline characteristics were well balanced and comparable with those of the intent-to-treat population. Both OS (median 11.9 vs. 7.1 months; HR 0.76; P = 0.373) and progression-free survival (median 7.2 vs. 5.2 months; HR 0.65; P = 0.224) were numerically longer and overall response rate (27% vs. 17%; P = 0.312) was numerically higher with nab-P + Gem vs. Gem. The most common grade ≥3 adverse events with nab-P + Gem vs. Gem were neutropenia (22% vs. 10%), fatigue (34% vs. 33%), and neuropathy (25% vs. 0%).
Conclusion
This subanalysis confirmed that nab-P + Gem is an efficacious treatment option and has a manageable safety profile in patients with MPC treated in Canada.
Trial registration
ClinicalTrials.gov identifier, NCT00844649.
Funding
Celgene Corporation, Summit, NJ, USA.
Similar content being viewed by others
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer-related mortality in Canada, the US, and Europe [1–3]. Estimates suggested that in 2015, 4800 Canadians were diagnosed with PC and 4600 died from the disease [1]. The 5-year relative survival ratio, which compares survival of patients with cancer to those without cancer, is 8% among Canadian patients with PC [1]. The observed 5-year survival rate for patients with PC is also 8%, a statistic that has not improved in the last 40 years [4]. Risk factors for the development of PC include smoking and obesity in addition to several well-established genetic mutations [5].
Surgery is the only potentially curative treatment for PC; however, surgical resection is an option in only 17% vs. 19% of Canadian PC cases [6]. Patients who are not eligible for surgery may be treated with systemic cancer therapy. For many years, gemcitabine (Gem) has been a standard treatment for metastatic PC (MPC) in Canada [4]. In addition, erlotinib is approved in Canada for the treatment of MPC, and the combination regimen of leucovorin, 5-fluorouracil (5-FU), irinotecan, and oxaliplatin (FOLFIRINOX) has been approved in some provinces [4]. The most recent regimen to gain approval for the treatment of MPC is nab-paclitaxel (nab-P) plus Gem [4, 7]. Since receiving this approval, nab-P + Gem has been evaluated by the pan-Canadian Oncology Drug Review [8] and has been deemed reimbursable by most but not all Canadian provinces [4].
Health Canada approved nab-P + Gem for the treatment of MPC based on the results of the phase III MPACT trial (N = 861; ClinicalTrials.gov identifier, NCT00844649), which compared nab-P + Gem vs. Gem alone [7]. In the MPACT trial, nab-P + Gem demonstrated superior efficacy in all trial endpoints, including the primary endpoint of overall survival (OS; median 8.5 vs. 6.7 months; hazard ratio [HR] 0.72; P < 0.001) [9]. An updated analysis revealed an even greater difference in median OS: 8.7 vs. 6.6 months (HR 0.72; P < 0.001) [10]. The combination of nab-P + Gem vs. Gem alone also demonstrated a longer progression-free survival (PFS; median 5.5 vs. 3.7 months; HR 0.69; P < 0.001) and higher overall response rate (ORR) by both independent (23% vs. 7%; P < 0.001) and investigator (29% vs. 8%; P < 0.001) review [9]. Grade ≥3 adverse events in the MPACT trial were effectively managed by dose reductions and treatment delays.
MPACT was a global trial that enrolled patients at 151 community and academic sites in North America (63%), Eastern Europe (15%), Australia (14%), and Western Europe (9%) [9]. Because cancer mortality rates vary among countries worldwide [11] (likely a reflection of differences in healthcare systems), clinical trials in oncology routinely stratify patient randomization by region. Geographic region was a stratification factor in the MPACT trial, as were performance status and presence or absence of liver metastases [9]. A significant treatment difference was observed for OS within the North American patient population, which consisted of patients primarily from the US (88%) and a small subset from Canada (12%). Additionally, a stepwise multivariate analysis revealed a lower risk of death for patients in North America, regardless of treatment [12]. Healthcare systems and treatment patterns are different between the US and Canada, and limited published information is available on the outcomes of Canadian patients treated with first-line nab-P + Gem. To understand how nab-P + Gem compared with Gem alone in Canadian patients, a subanalysis of the MPACT trial was undertaken to compare efficacy, safety, and treatment exposure in the subgroup of patients treated in Canada.
Methods
The MPACT trial was approved by the independent ethics committee at each participating institution and was conducted in accordance with the International Conference on Harmonisation E6 requirements for Good Clinical Practice [9]. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Patients
Study design and patient characteristics for the phase III MPACT trial have been described previously [9]. Briefly, adults (≥18 years of age) with a Karnofsky performance status (KPS) ≥70, no prior chemotherapy received for metastatic disease, and histologically or cytologically confirmed MPC as assessed by Response Evaluation Criteria In Solid Tumors (RECIST; version 1.0) [13] were included in this analysis. Patients were permitted to have received 5-FU or Gem as a radiation sensitizer in the adjuvant setting, provided that treatment was received ≥6 months prior to randomization. Adequate hematologic, hepatic, and renal function was also required.
Study Design
Patients were randomized 1:1 to receive (1) intravenous nab-P 125 mg/m2 followed by intravenous Gem 1000 mg/m2 on days 1, 8, 15, 29, 36, and 43 of an 8-week cycle (cycle 1), then on days 1, 8, and 15 every 4 weeks for each subsequent cycle or (2) Gem 1000 mg/m2 weekly for the first 7 weeks of an 8-week cycle (cycle 1), then for the first 3 weeks of a 4-week cycle (cycle ≥2). Patients were stratified according to KPS, presence of liver metastases, and geographic region. Patients were treated until either disease progression or unacceptable toxicity. Tumor response was evaluated every 8 weeks using spiral computed tomography or magnetic resonance imaging. At baseline and every 8 weeks thereafter, serial measurements of carbohydrate antigen 19-9 (CA 19-9) levels were obtained. The primary endpoint was OS, and secondary endpoints were PFS and ORR, as assessed by independent radiological review according to RECIST version 1.0. Also examined was OS as a function of specific decreases in CA 19-9 levels (20% and 60%). Safety was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 [14], and summarized according to the Medical Dictionary for Regulatory Activities, version 15.0 [15].
Statistical Analyses
Efficacy analyses were performed on all randomized patients in the Canadian cohort. The Kaplan–Meier method was used to determine OS, and statistical significance was assessed with a log-rank test. A stratified Cox proportional hazards model was used to determine the associated HR and two-sided 95% confidence intervals (CIs). For patients who were lost to follow-up, survival data were censored at the last date that they were known to be alive. The original cutoff for OS analysis was September 17, 2012. A nonstratified log-rank test was PFS between the treatment arms, and the HR and two-sided 95% CIs were estimated by a Cox proportional hazards model. Differences in ORR were assessed by χ 2 test.
Results
Baseline Characteristics
A total of 63 patients (33 receiving nab-P + Gem and 30 receiving Gem alone) were randomized for treatment at 7 Canadian centers (average number of patients per center was 4.7 for nab-P + Gem and 4.3 for Gem alone). In general, baseline characteristics were well balanced between the 2 treatment arms in the Canadian cohort and were similar to those of the intent-to-treat (ITT) population from the MPACT trial; however, some differences were noted (Table 1) [9]. The Canadian cohort included a greater percentage of male patients, patients with ≥3 metastatic sites, and patients with a biliary stent compared with the ITT population. Fewer patients in the Canadian cohort had a KPS of 100 or previous Whipple procedure than in the ITT population. In the Canadian cohort, there was a greater percentage of patients whose primary tumor was located in the head of the pancreas in the nab-P + Gem arm compared with the Gem-alone arm.
Efficacy
Overall Survival in the Canadian Population
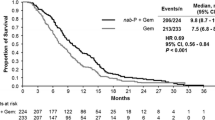
The OS data in the Canadian population were based on 47 deaths (75% of the population), including 24 in the nab-P + Gem arm (73%) and 23 in the Gem-alone arm (77%). In the Canadian cohort, OS was numerically longer with nab-P + Gem vs. Gem alone (median 11.9 vs. 7.1 months; HR 0.76; 95% CI 0.41–1.40; P = 0.373; Fig. 1). Kaplan–Meier estimates of OS at 24 months following randomization were 15% for nab-P + Gem and 9% for Gem alone.
Progression-Free Survival
For the PFS analysis by independent radiological review, 38 patients in the Canadian cohort (60%) either had progressive disease or had died, including 23 (70%) in the nab-P + Gem arm and 15 (50%) in the Gem-alone arm. In the Canadian cohort, PFS was numerically longer in patients treated with nab-P + Gem vs. Gem alone (median 7.2 vs. 5.2 months; HR 0.65; 95% CI 0.32–1.31; P = 0.224; Table 2).
Overall Response Rate
The independently assessed ORR in the Canadian cohort was numerically higher in patients treated with nab-P + Gem vs. Gem alone (27% vs. 17%; response rate ratio, 1.64; 95% CI 0.62–4.34; P = 0.312; Table 2). No Canadian patient in either treatment arm achieved a complete response. The disease control rate (partial response + stable disease [for ≥16 weeks]) was also numerically higher in Canadian patients treated with nab-P + Gem than in patients treated with Gem alone (58% vs. 37%; response rate ratio, 1.57; P = 0.097).
Evaluation of CA 19-9 Levels
Patients in the Canadian cohort were evaluated for CA 19-9 levels at baseline and every 8 weeks. Assessment of OS in relation to CA 19-9 decrease (both 20% and 60%) was based on the 39 patients who had a measurement at baseline and ≥1 time point after baseline. In a pooled analysis of these 39 patients (which included both treatment groups), OS was significantly longer in Canadian patients who had a ≥20% CA 19-9 decrease from baseline to nadir compared with those who had a <20% decrease (median 14.5 vs. 7.8 months; HR 0.28; 95% CI 0.12–0.68; P = 0.003; Table S1). Similarly, OS was significantly longer in patients with a ≥60% CA 19-9 decrease from baseline to nadir than in those with a <60% decrease (median 15.7 vs. 9.2 months; HR 0.40; 95% CI 0.17–0.91; P = 0.025). The maximum absolute percentage reductions in CA 19-9 from baseline (median) were 87.3% and 78.2% for patients in the nab-P + Gem and Gem-alone arms, respectively.
Treatment Exposure and Secondary Therapy Use
In general, treatment duration was slightly longer, but cumulative doses were similar in the Canadian subgroup compared with the ITT population. The median treatment duration was 4.1 months (range 0.3–20.7) in patients treated with nab-P + Gem and 3.1 months (range 0.6–16.6) in patients treated with Gem alone (Table 3). In the nab-P + Gem arm, 50% of patients had ≥1 nab-P dose reduction and 38% had ≥1 Gem dose reduction. Forty percent of patients in the Gem-alone arm had ≥1 dose reduction. In the nab-P + Gem arm, the median percentage of per-protocol doses given was 75.0% and 81.2% for nab-P and Gem, respectively. The median percentage of per-protocol dose given in the Gem-alone arm was 85.0%. For patients treated with nab-P + Gem, the median nab-P dose intensity was 68.4 mg/m2/week, and the median Gem dose intensity was 627.8 mg/m2/week. The median dose intensity for Gem alone was 667.3 mg/m2/week.
Fewer patients in the nab-P + Gem arm received a subsequent therapy than in the Gem-alone arm (30% vs. 43%; Table 4). In both treatment arms, 5-FU- or capecitabine-based regimens were the most commonly used secondary therapies.
Safety
The most common grade ≥3 nonhematologic adverse events were fatigue and peripheral neuropathy, which occurred in 34% and 25% of patients treated with nab-P + Gem and 33% and 0% of patients treated with Gem alone, respectively (Table 5). Neutropenia was the most common grade ≥3 hematologic adverse event in both treatment arms (22% for nab-P + Gem and 10% for Gem alone). The rates of grade ≥3 anemia, thrombocytopenia, and leukopenia were similar between the 2 treatment arms. Grade ≥3 vomiting was slightly more common in the Gem-alone arm vs. nab-P + Gem arm (13% vs. 6%).
Discussion
This subanalysis of the MPACT trial evaluated the efficacy and safety outcomes with nab-P + Gem vs. Gem alone in patients treated in Canada. The results in the Canadian cohort were consistent with those in the ITT population, in which nab-P + Gem was associated with improved OS, ORR, and PFS compared with Gem alone [9]. A survival difference of nearly 5 months was observed between the nab-P + Gem and Gem-alone arms in the Canadian subpopulation (median 11.9 vs. 7.1 months); however, this difference did not reach statistical significance. In addition, the 7% of patients treated in Canada had numerically longer OS and PFS and a higher ORR than patients globally in the ITT population, especially those receiving nab-P + Gem, although the outcomes could not be compared statistically given that the Canadian patients were a small subset and such a comparison was not planned in the trial protocol.
In general, treatment exposure and secondary therapy use were similar in the Canadian and ITT populations [9]; however, fewer Canadian patients treated with nab-P + Gem received a subsequent therapy than ITT patients treated with nab-P + Gem. Patients in the Canadian cohort had a lower incidence of hematologic adverse events than those in the ITT population, but Canadian patients had higher incidences of fatigue (both treatment arms) and peripheral neuropathy (nab-P + Gem only) than ITT patients.
An examination of baseline characteristics and treatment exposure did not reveal the reasons for the efficacy observed in the Canadian population. In fact, the Canadian cohort had higher rates of certain baseline characteristics that might be expected to be associated with worse efficacy, including the percentages of patients who were male, had ≥3 sites of metastasis, or had a KPS <100 [11, 16–19]. Furthermore, as described herein, the treatment exposure in the Canadian cohort was similar to that in the ITT population, and the rates of secondary therapy use were similar or slightly lower than in the ITT population. Conversely, there was a higher rate of patients per treatment center in the Canadian cohort (63 patients/7 centers = 9 patients/center) vs. the ITT population (861 patients/151 centers = 5.7 patients/center), suggesting the possibility that study physicians in the Canadian cohort could have become more familiar with the per-protocol treatments. The potential effect of this familiarity on efficacy and tolerability is difficult to quantify.
In the MPACT trial, there was a lower risk of death for patients in North America, regardless of treatment. Whether unique features of the Canadian healthcare system might have influenced efficacy outcomes among patients in the MPACT trial is an interesting question. Canadian citizens have access to universal healthcare through the single-payer system; thus, all patients receive the same level of care. Universal healthcare may have allowed Canadian patients as a whole to receive more uniform access to medical visits, supportive care medications, and second-line therapies. The universal healthcare system also provides educational awareness programs and special care for those in need, including the elderly. The impact of these differences on treatment effectiveness at this point is speculative.
This subanalysis was subject to a number of limitations. This was an unplanned, post hoc analysis; no consideration in the study design was given to allowing for statistical comparisons within the cohort. In addition, this analysis included only 63 patients; this small sample size was likely the factor that prevented numerous treatment arm comparisons from reaching statistical significance. Finally, some differences were apparent between the treatment arms in the Canadian cohort of patients, including the higher percentage with tumors in the head of the pancreas in the nab-P + Gem arm vs. the Gem-alone arm at baseline and the higher percentage in the Gem-alone arm who received secondary therapies. These limitations must be kept in mind when interpreting the results of the study.
Health Canada has approved nab-P + Gem and FOLFIRINOX for the treatment of patients with MPC based on the phase III MPACT and PRODIGE trials (ClinicalTrials.gov identifier, NCT00112658), respectively, in which nab-P + Gem and FOLFIRINOX demonstrated superior efficacy vs. Gem alone [4, 9, 20]. However, there is little clinical evidence to guide treatment selection between these regimens. Direct comparisons across the trials are not appropriate given differences in patient populations. A recent retrospective analysis investigated the likelihood of meeting eligibility criteria for the MPACT and PRODIGE trials and possible associations with efficacy among patients with MPC who were treated with Gem at British Columbia regional cancer centers between January 2000 and December 2011 [21]. The analysis found that 45% and 25% of the analyzed cohort would have been eligible to receive nab-P + Gem and FOLFIRINOX, respectively. Performance status and elevated bilirubin level were the most frequent reasons for ineligibility regardless of whether MPACT or PRODIGE criteria were applied. Furthermore, the study suggested that simply meeting eligibility criteria had a dramatic impact on efficacy outcomes: even though all patients were treated with Gem, those who met the more stringent criteria of the PRODIGE trial had a longer median OS than those who met the more inclusive criteria of the MPACT trial (8.6 vs. 6.7 months). These data suggest a substantial correlation between eligibility criteria and efficacy, illustrating the importance of randomized trials for comparing treatments and avoiding cross-trial comparisons.
Conclusions
This subanalysis confirmed that nab-P + Gem is an efficacious treatment option for Canadian patients with MPC and has a manageable safety profile. The consistency of the findings in the overall treatment population lends support to their overall implications, despite certain limitations, such as the small sample size. Additional data on the efficacy and safety of nab-P + Gem in Canadian patients are likely to become increasingly available as physicians continue to use the regimen to treat their patients with MPC.
References
Canadian Cancer Society. Canadian cancer statistics. 2015. Available at: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2015-EN.pdf. Accessed Nov 9, 2015.
American Cancer Society. Cancer facts and figures. 2015. Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed Nov 9, 2015.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403.
Pancreatic Cancer Canada. Know the facts and statistics. Available at: http://www.pancreaticcancercanada.ca/site/PageServer?pagename=facingpancreaticcancer_facts. Accessed Nov 9, 2015.
Chiorean EG, Coveler AL. Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies. Drug Des Devel Ther. 2015;9:3529–45.
Hurton S, MacDonald F, Porter G, Walsh M, Molinari M. The current state of pancreatic cancer in Canada: incidence, mortality, and surgical therapy. Pancreas. 2014;43:879–85.
Cancer Care Ontario. nab-Paclitaxel drug monograph. 2015.
Pan-Canadian Oncology Drug Review. Pan-Canadian oncology drug review final economic guidance report: nab-paclitaxel (Abraxane) for pancreatic cancer. Available at: https://www.cadth.ca/sites/default/files/pcodr/pcodr-abraxane-mpc-fn-egr.pdf. Accessed Nov 9, 2015.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Goldstein D, El-Maraghi RH, Hammel P, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413.
World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed Sept 17, 2015.
Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20:143–50.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81.
MedDRA Maintenance and Support Services Organization. Introductory Guide to MedDRA Version 15.0. Chantily: MedDRA Maintenance and Support Services Organization; 2012. http://www.meddra.org/sites/default/files/guidance/file/intguide_15_0_english.pdf. Accessed Mar 24, 2016.
Kou T, Kanai M, Yamamoto M, et al. Prognostic model for survival based on readily available pretreatment factors in patients with advanced pancreatic cancer receiving palliative chemotherapy. Int J Clin Oncol. 2016;21:118–25.
Blank PR, Szucs TD. Pancreas cancer: influence of gender and other demographic and clinical parameters on survival [abstract 200]. J Clin Oncol. 2014;32(suppl 3).
Xue P, Zhu L, Wan Z, et al. A prognostic index model to predict the clinical outcomes for advanced pancreatic cancer patients following palliative chemotherapy. J Cancer Res Clin Oncol. 2015;141:1653–60.
Bolm L, Janssen S, Kasmann L, et al. Predicting survival after irradiation of metastases from pancreatic cancer. Anticancer Res. 2015;35:4105–8.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Peixoto RD, Ho M, Renouf DJ, et al. Eligibility of metastatic pancreatic cancer patients for first-line palliative intent nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Am J Clin Oncol. 2015. [Epub ahead of print].
Acknowledgments
Sponsorship for this study and article processing charges was funded by Celgene Corporation, Summit, NJ, United States. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Editorial assistance in the preparation of this manuscript was provided by Aaron Runkle, PhD, of MediTech Media. Support for this assistance was funded by Celgene Corporation. The authors were fully responsible for all content and editorial decisions of this manuscript.
Disclosures
Scot Dowden has participated in advisory boards as a speaker for Amgen, Bayer, Celgene, Lilly, and Roche. Hagen Kennecke has received honoraria and research support from Amgen and honoraria from Celgene and Novartis. Helen Liu is an employee and stockholder of Celgene. Robert El-Maraghi has participated in advisory boards as a speaker and has received honoraria from Celgene and Lilly, and has participated in advisory boards and received travel support and honoraria from Roche. Alfredo Romano is an employee and stockholder of Celgene. Mustapha Tehfe has participated in advisory boards for Celgene, Lilly, Ipsen, Merck, and Novartis and has been a speaker for Celgene, Lilly, Amgen, and Novartis. Bernard Lesperance, Felix Couture, and Richard Letourneau declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/3E84F06021D67FC0.
An erratum to this article is available at http://dx.doi.org/10.1007/s12325-016-0442-2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tehfe, M., Dowden, S., Kennecke, H. et al. nab-Paclitaxel Plus Gemcitabine Versus Gemcitabine in Patients with Metastatic Pancreatic Adenocarcinoma: Canadian Subgroup Analysis of the Phase 3 MPACT Trial. Adv Ther 33, 747–759 (2016). https://doi.org/10.1007/s12325-016-0327-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0327-4