Abstract
Introduction
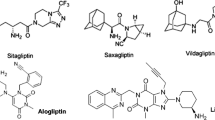
Use of dipeptidyl peptidase-4 (DPP-4) inhibitors is prevalent for the treatment of type 2 diabetes since they have fewer adverse effects compared with other non-insulin medications currently available; however, as monotherapy, the glycosylated hemoglobin (HbA1c)-lowering power of these agents is moderate. The aim of this article is to evaluate the current literature regarding the safety and efficacy of DPP-4 inhibitors in combination with metformin.
Methods
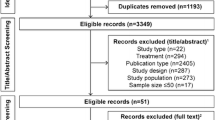
A literature search was conducted through MEDLINE (from 1950 to October 2012), PubMed (from 1966 to October 2012), EMBASE (from 1966 to October 2012), and International Pharmaceutical Abstracts (from 1970 to October 2012) using the search terms “sitagliptin,” “linagliptin,” “alogliptin,” “vildagliptin,” “saxagliptin,” and “metformin.” Studies that did not evaluate the DPP-4 inhibitors in combination with metformin and those that were not phase 3, were excluded.
Results
Many of the studies evaluated DPP-4 inhibitors in combination with metformin versus glucagon-like peptide-1 (GLP-1) agonists, placebo, DPP-4 inhibitors as monotherapy, thiazolidinediones, and sulfonylureas. The results of these noninferiority trials were that DPP-4 inhibitors as a whole are noninferior to either each other or other agents except for GLP-1 agonists. Also, in superiority studies, GLP-1 agonists proved to have greater HbA1c lowering.
Conclusion
In summary, DPP-4 inhibitors play a vital role in the treatment of diabetes. They have relatively limited adverse effects, especially regarding hypoglycemia. DPP-4 inhibitors in combination with metformin are generally well tolerated and are available as combination products to reduce pill burden and enhance compliance. The limitations to DPP-4 inhibitors are the lack of outcomes data and more limited HbA1c lowering than other medications currently approved for the treatment of type 2 diabetes. However, as previously stated, thiazolidinediones, glinides, sulfonylureas, pramlinitide, and GLP-1 agonists are all quite beneficial in HbA1c lowering but are not without major adverse effects. Therefore, DPP-4 inhibitors have a vital role as an oral add-on agent for the treatment of type 2 diabetes.
Similar content being viewed by others
References
Januvia [package insert]. Northumberland, United Kingdom: Merck & Co., Inc.
Onglyza [package insert]. Princeton, New Jersey: Bristol-Myers Squibb; December 2011.
Tradjenta [package insert]. Ridgefield, Connecticut: Boehringer Ingelheim Pharmaceuticals, Inc; September 2012.
Nesina [package insert]. Deerfield, Illinois: Takeda Pharmaceuticals America, Inc; January 2013.
Galvus [prescribing information]. Nurenberg, Germany: Novartis Pharmaceuticals.
Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association; European Association for Study of Diabetes. Medical Management of Hyperglycemia in Type 2 Diabetes: A consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193–203.
Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endoc Pract. 2009;15:540–559.
Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association; European Association for Study of Diabetes. Management of hyperglycemia in type 2 diabetes: A patient centered approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;25:1364–1379.
CONSORT. CONSORT 2010 checklist of information to include when reporting a randomized trial. Available at: http://www.consort-statement.org/consort-statement/. Accessed Jan 15 2013.
Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of noninferiority and equivalence randomized trials extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–2604.
Wainstein J, Katz L, Engel SS, et al. Initial therapy with the fixed-dose combination of sitagliptin and metformin results in greater improvement in glycaemic control compared with pioglitazone monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14:409–418.
Arechavaleta R, Seck T, Chen Y, et al. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2011;13:160–168.
Pratley R, Nauck M, Bailey T, et al; 1860-LIRADPP-4 Study Group. One year liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomized, parallel-group, open-label trial. Int J Clin Pract. 2011;65:397–407.
Scheen AJ, Charpentier G, Ostgren CJ, Hellqvist A, Gause-Nilsson I. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2010;26:540–549.
Bergenstal RM, Wysham C, MacConell L, et al; DURATION-2 Study Group. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomized trial. Lancet. 2010;376:431–439.
Scott R, Loeys T, Davies MJ, Engel SS. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:959–969.
Gallwitz B, Rosenstock J, Rauch T, et al. 2-Year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomized, double-blind, non-inferiority trial. Lancet. 2012;380:475–483.
Haak T, Meinicke T, Jones R, Weber S, Eynatten M, Woerle Hj. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2012;14:565–574.
Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:65–74.
Filozof C, Gautier JF. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with Type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study. Diabet Med. 2010;27:318–326.
Blonde L, Dagogo-Jack S, Banerji MA, et al. Comparison of vildagliptin and thiazolidinedione as add-on therapy in patients inadequately controlled with metformin: results of the GALIANT trial — a primary care, type 2 diabetes study. Diabetes Obes Metab. 2009;11:978–986.
Ferrannini E, Fonseca V, Zinman B, et al. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157–166.
Goke B, Gallwitz B, Eriksson J, Hellqvist A, Nilsson-Gause I. Saxagliptin is non-inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52 week randomized controlled trial. Int J Clin Pract. 2010;64:1619–1631.
Defronzo RA, Hissa MN, Garber AJ, et al; Saxagliptin 014 Study Group. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649–1655.
Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy; a multicentre, randomized, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46–55.
Takeda Pharmaceuticals. Takeda resubmits new drug applications to the United States Food and Drug Administration for alogliptin and the fixeddose combination alogliptin and pioglitazone. Available at: http://www.takeda.us/newsroom/press_release_detail.aspx?year=2012&id=244. Accessed Jan 22 2013.
US Food and Drug Administration. FDA approves three new drug treatments for type 2 diabetes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm336942.htm. Accessed Jan 28 2013.
Merck. Putting patients first by addressing significant unmet medical needs: update on cardiovascular outcomes study with Januvia. Available at: http://www.merck.com/mrl/clinical_trials/outcomes_study.html. Accessed March 21 2013.
ClinicalTrials.gov. Sitagliptin cardiovascular outcome study (0431-082 AM1) (TECOS). Available at: http://clinicaltrials.gov/ct2/show/NCT00790205?term=DPP+4+inhibitors&rank=132. Accessed March 21 2013.
ClinicalTrials.gov. Does Saxagliptin Reduce the Risk of Cardiovascular Events When Used Alone or Added to Other Diabetes Medications (SAVOR-TIMI 53). Available at: http://clinicaltrials.gov/ct2/show/NCT01107886?term=saxagliptin&cond=%22Coronary+Artery+Disease%22&rank=2. Accessed March 21 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
To view enhanced content go to www.advancesintherapy.com
Rights and permissions
About this article
Cite this article
Fass, A.D., Gershman, J.A. Efficacy and Safety of Dipeptidyl Peptidase-4 Inhibitors in Combination with Metformin. Adv Therapy 30, 337–353 (2013). https://doi.org/10.1007/s12325-013-0023-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0023-6