Abstract
We reviewed CD56 expression on bone marrow (BM) granulocytes and monocytes by flow cytometry in 101 cases of myeloproliferative neoplasm (MPN) and compared them with those from 42 cases of myelodysplastic syndrome (MDS), 47 cases of negative BM after stem cell transplantation, and 24 cases of negative BM after chemotherapy. Forty cases of negative staging BM for lymphoma were used as negative controls. CD56 expression on granulocytes was also compared with corresponding morphologic and molecular genetic findings in five patients who underwent serial BM sampling. Using 10 % as positive threshold, aberrant CD56 expression was detected on granulocytes and monocytes in 20 and 34 % cases, respectively, in MPN, and in 18 and 36 % cases, respectively, in high-grade MDS. Aberrant CD56 expression was present in all subtypes of MPN with the highest frequency seen in primary myelofibrosis (37 % on granulocytes, 40 % on monocytes). Compared to monocytes, granulocytes had lower frequencies of CD56 expression but higher specificity for abnormal cells. Low levels of CD56 expression were detected on the granulocytes in normal BM after chemotherapy and stem cell transplantation, but the expression levels were generally below 10 %. In the patients with serial samples, CD56 expression paralleled with disease status by BM morphology, BCR/ABL1 transcripts, or percentage of BM recipient cells. We conclude that aberrant CD56 expression on granulocytes is present in a subset of patients in all subtypes of MPN. Identification of abnormal CD56-positive granulocytes is helpful in both initial diagnosis and follow-up of patients in MPN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myeloproliferative neoplasm (MPN) is a group of malignant stem cell neoplasm characterized by clonal proliferation of bone marrow hematopoietic cells. Diagnoses of these entities require a combination of morphology, immunophenotype, genotype, and clinical presentation [1]. Flow cytometry phenotyping is widely used as an ancillary tool in the diagnoses of these diseases. Flow cytometry has traditionally played a role in defining phenotype and estimating percentage of blast population. More recently, phenotypic aberrancies in cell populations other than blasts such as granulocytes, monocytes, and erythroid precursors have been observed [2, 3]. Abnormal granularity and maturation in granulocytes, aberrant expression of antigens such as CD56 in granulocytes and monocytes, and abnormal expression of CD71 and CD117 on erythroid precursors were well recognized as aberrant expression patterns. However, much of the emphasis was placed on delineating abnormal phenotypes of myelodysplastic syndrome (MDS), while similar studies on MPN were rare [4, 5].
CD56 is one of the many antigens frequently used to assess neoplastic population in hematologic malignancies. CD56 is an adhesion molecule most often expressed in neural tissue and on large granular lymphocytes. Aberrant CD56 expression can be seen in approximately 24 % of acute myeloid leukemia, 14 % of acute promyelocytic leukemia, and 70–80 % multiple myeloma [6, 7]. In myeloid malignancies, aberrant CD56 antigen expression on blasts can be seen in all types of acute and chronic myeloid neoplasms [8, 9]. Using cluster analysis and specifically constructed antibody panel targeting on blast population, aberrant expression of various antigens, including CD56, was detected on blasts in chronic myeloid neoplasms [9]. High frequency of CD56 expression has been reported on monocytic cells in both acute and chronic monocytic leukemia and the expression of CD56 is widely accepted as an aberrant marker to define abnormal monocytes in these diseases [10–12].
Despite the well-defined roles of CD56 in assessing abnormal populations in acute myeloid leukemia and myelodysplastic syndrome, its value in MPN is less certain. In MPN, significant increase of blasts or monocytes may often be absent. Rather, proliferations of granulocytic and erythroid lineages are dominant features. Analysis of aberrant expression in blasts and monocytes may require specially constructed panel to discriminate these populations [9]. Information on aberrant CD56 antigen expression on granulocytes is limited and contradictory in the literatures [4, 5]. In addition, some authors suggested that CD56 expression may not be completely specific for defining abnormal populations. Expression of CD56 may be present on normal granulocytes following chemotherapy and growth factor treatment [13]. To better understand the nature of CD56 in the diagnosis of MPN, here in a single institution study, we analyzed CD56 expression in all subtypes of MPN in granulocytes and monocytes, and compared the results with patients with myelodysplastic syndrome and normal bone marrows after chemotherapy and bone marrow transplantation. We also analyzed changes of CD56 expression in serial samples and compared the results with bone marrow morphology and genetic information.
Materials and methods
Subjects
This study was approved by the institutional review board at Thomas Jefferson University. The cases were retrieved from the pathology file of Thomas Jefferson University within the past 7-year period (2006 to 2012). All cases had bone marrow specimens with aspirate and biopsy for morphologic examination and aspirate for flow cytometry analysis. Selected cases had cytogenetic and molecular studies. The diagnosis of MPN and MDS was based on the criteria of the 2008 WHO classification [1]. Categories of MPN included chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), and MPN unclassified (MPN-U). Categories of MDS included low-grade MDS (refractory cytopenia with unilineage dysplasia and refractory anemia with ringed sideroblasts) and high-grade MDS (refractory anemia with excess blasts-1, refractory anemia with excess blasts-2, refractory cytopenia with multilineage dysplasia). The normal control cases were negative staging bone marrows for lymphoma. The posttreatment bone marrows included morphologically normal bone marrows from patients who received chemotherapy or bone marrow transplantation for hematologic malignancies.
Flow cytometry analysis
Samples for flow cytometry were obtained during the bone marrow biopsy procedure and submitted directly to the laboratory for analysis within 24 h. Bone marrow aspirate specimens were subjected to FACS Lysing Solution treatment (BD Biosciences, San Jose, CA) to eliminate mature red blood cells. Four-color combinations of fluorescein isothiocyanate, phycoerythrin, peridinin–chlorophyll–protein, or allophycocyanin-conjugated monoclonal antibodies (Becton Dickinson, Franklin Lakes, NJ) for CD2 (S5.2), CD3 (SK7), CD4 (SK3), CD5 (L17F12), CD7 (4H9), CD8 (SK1), CD10 (HI10a), CD13 (L138), CD14 (MфP9), CD16 (NKP15), CD19 (SJ25C1), CD20 (L27), CD22 (S-HCL-1), CD23 (EBVCS-5), CD33 (P67.6), CD34 (My10), CD38 (HB7), CD45 (2D1), CD56 (NCAM16.2), CD57 (HNK-1), CD117 (104D2), HLA-DR (L243), Kappa (TB28-2), and Lambda (1-155-2) were utilized. The four-color tube configuration included the following: CD45/CD57/CD56/CD3, CD45/CD64/CD2/CD71, CD45/CD10/CD22/CD34, CD45/CD16/CD13/CD33, CD45/CD20/CD23/CD5, CD45/CD3/CD4/CD8, CD45/CD7/CD38/HLA-DR, CD45/CD34/CD117/CD14, and CD45/Kappa/Lambda/CD19. Isotype controls (Becton Dickinson, Franklin Lakes, NJ) IgG1 (X40) and IgG2 (X39), and biological negative cells within each tube were used as negative controls. The stained cells were acquired on a bench top flow cytometer (FACScanto or FACScalibur, Becton Dickinson) and analyzed using Kaluza software (Beckman Coulter, Fullerton, CA). Daily calibration was performed using CaliBRITE Beads (Becton Dickinson). The fluorescence intensity was measured using a logarithmic scale with signal intensity ranging from 100 to 104.
Granulocytes and monocytes were isolated based on CD45 and side scatter characteristics. Care was taken to exclude carryover of other populations (blasts, mature lymphocytes, hematogones) within the granulocyte and monocyte gates. CD56-positive natural killer cells and T cells were frequently back-gated to ensure that these cells were not included in the monocyte gates. CD56 positivity on granulocytes and monocytes was determined based on isotype controls. CD56 expression was measured based on percentage of positive cells.
Quantitative polymerase chain reaction for BCR/ABL1 and bone marrow engraftment study
RNA was extracted from bone marrow aspirates collected in EDTA tube. Extracted RNA was subjected to real-time reverse transcription-polymerase chain reaction (RT-PCR) to amplify three BCR/ABL1 fusion transcripts: b2a2, b3a2, and e1a2. An additional amplification of the ABL1 gene was performed as a control for sample RNA quality and as a reference for relative quantification. If there was no amplification of fused mRNA (BCR/ABL1 ratio = 0), the result was reported as negative. For samples with positive results, the ratio between the quantities of the fused mRNA (BCR/ABL1) and control mRNA (ABL1) was reported.
Bone marrow engraftment study was performed using standard methods according to the manufacturer instructions (Applied Biosystems, Foster City, CA). Genomic DNA isolated from bone marrow specimens was subjected to PCR amplification of 15 microsatellite loci using the AmpFlSTR Profiler kit or AmpFlSTR Identifiler kit (Applied Biosystems). One microliter of PCR products and 9 μL of deionized formamide/GeneScan-500 (ROX) for Profiler and deionized formamide/GeneScan-500 (LIZ) for Identifiler (Applied Biosystems) were mixed according to the manufacturer protocol, heated at 95 °C for 2 min, and placed on ice for at least 1 min before electrokinetic injection on the ABI 3100 or 3130 capillary electrophoresis instrument (Applied Biosystems). Post-transplant chimerism was calculated using an average of at least three informative loci.
Statistical analysis
Statistical analysis was performed using the Stratview software from the SAS institute (SAS Institute Inc. Cary, NC). The data were analyzed with chi square test. A p value of <0.05 was considered as statistically significant.
Results
Patient characteristics
The study group consisted of 101 cases of MPN, which included 33 cases of CML, 20 cases of ET, 8 cases of PV, 27 cases of PMF, and 13 cases of MPN-U. The ages of patients, WBC counts, hemoglobin concentrations, and platelet counts for each subtype of MPN are listed in Table 1. Additional 24 cases of post-chemotherapy, 47 cases of post-bone marrow transplantation, and 42 cases of MDS were also included in the study. Forty cases of negative bone marrow for lymphoma staging were included as negative controls.
CD56 expression on granulocytes
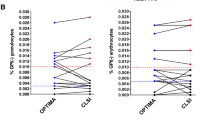
CD56 was expressed on less than 2 % of granulocytes in all 40 negative control cases. Using 10 % as cutoff value, CD56 was positive on granulocytes in 15 % CML, 10 % ET, 13 % PV, 37 % PMF, and 15 % MPN-U (Table 2). The CD56-positive rates of granulocytes varied among the subtypes of MPNs. PMF had the highest percentage of CD56 expression among all subtypes of MPN, and the differences were statistically significant from ET (p = 0.0356), but not from PV (p = 0.387), CML (p = 0.051), and MPN, NOS (p = 0.271). Among the 20 cases of MPN with increased CD56 expression on granulocytes, 18 cases showed co-expression of CD56 on monocytes. In MDS cases, CD56 expression on granulocytes was not detected in low-grade MDS, but was positive in 18 % of high-grade MDS (Fig. 1).
Change of CD56 expression on granulocytes in MPN patients after treatment
Serial follow-up specimens were available in five patients (six serial samples), which included two patients with CML, two patients with PMF, and one patient with PV. Three or more consecutive specimens were available from all five patients. All patients had CD56-positive granulocytes and positive molecular and morphologic evidence of disease at the initial biopsy.
CD56 expression on granulocytes and quantitative BCR/ABL1 expression were monitored on serial specimens from two CML patients in a period of 9 and 27 months, respectively. The first patient showed complete concordance of CD56 reduction on granulocytes with the reduction of BCR/ABL1 transcripts following treatment. In the second patient, CD56 level paralleled with BC R/ABL1 transcripts during the disease course (Fig. 2a). CD56 expression on granulocytes was compared with bone marrow engraftment results in two patients who received bone marrow transplantation (one CML and one PMF). Percent of recipient cells and CD56 expression on granulocytes were monitored in a series of time points pre- and post-transplant. CD56 expression showed complete match to the engraftment results on these two patients (Fig. 2b). In the final two patients, CD56 expression was compared with bone marrow morphology. Patient 4 was initially diagnosed as ET and the disease progressed into myelofibrosis in the period of 3 years. CD56 expression on granulocytes ranged between 5 and 18 % and was concordant with the morphological evidence of persistent diseases. Patient 5 was diagnosed as PMF and achieved remission after therapy in approximately 1 year of follow-up. CD56 expression was detected in the initial diagnostic marrow and was negative in several subsequent specimens when the disease was in remission by bone marrow morphology.
Comparison of CD56 expression on granulocytes with genetic and morphologic results in serial specimens. a Serial specimens from a CML patient were analyzed for CD56 expression on granulocytes and BCR/ABL transcripts by quantitative PCR. The percentage of CD56+ granulocytes closely paralleled with BCR/ABL transcripts during the disease course. b Serial specimens from a patient with primary myelofibrosis were analyzed for CD56 expression on granulocytes before and after bone marrow transplantation. The results were compared with the engraftment studies. CD56+ granulocytes were closely associated with the presence/absence of recipient cells before and after BM transplantation
CD56 expression in patients with history of chemotherapy and bone marrow transplantation
Normal bone marrows from 24 patients who previously received chemotherapy and 47 patients who underwent bone marrow transplantation (BMT) were evaluated for CD56 expression. One case of post-chemotherapy and one case of post-BMT patients showed positive CD56 expression. The percentages of CD56-positive granulocytes from both patients were relatively low (Table 3). In addition, 38 % cases of post-chemotherapy and 36 % cases of post-BMT samples showed between 2 and 10 % CD56-positive granulocytes.
CD56 expression on monocytes
CD56 expression on monocytes was analyzed and compared with expression on granulocytes. CD56 was expressed on less than 2 % of monocytes in all 40 negative control cases (Fig. 1). Using same positive criteria as on granulocytes (10 % positive as the cutoff value), CD56-positive monocytes were observed on all categories of MPN and high-grade MDS (Table 2). In general, the intensity of CD56 was higher on monocytes than on granulocytes (data not shown). PMF and PV appeared to have slightly higher percentage of CD56-positive cases (Table 2); however, the differences were not statistically significant between PMF and PV to the other categories (p > 0.05). Similar to MPN, higher intensity of CD56 expression was observed on monocytes than on granulocytes in MDS (Table 3).
Discussion
CD56 is well defined as an aberrant antigen expressed on blast populations in myeloid neoplasms. Mann et al. analyzed CD56 expression in a series of acute and chronic myeloid leukemias, and found CD56 expression on blasts in acute myeloid leukemia and chronic myeloid leukemia and MDS when blasts were increased [8]. In chronic myeloid leukemia without an increase of blasts, aberrant CD56 expression can be assessed using custom-designed antigen panels by paring of CD56 with a stem cell marker. Using a four-color flow cytometry panel, Harrington et al. analyzed aberrant antigens on blast population by paring CD34 with various target antigens. Aberrant CD56 expression, along with other variant antigenic patterns, was detected on blasts despite low percentages (often below 1 %) in most of the studied cases [9]. Although the value of CD56 expression in blast population is well established, the utility of aberrant CD56 expression in non-blast populations is poorly defined due to limited information from previous studies [4]. In addition, some authors suggested that CD56 could be upregulated in normal granulocytes and monocytes after chemotherapy and growth factor treatment, and therefore might not be completely specific to define abnormal populations in granulocytes and monocytes [13].
To address these issues, we analyzed CD56 expression in a series of MPN including all subtypes. Since CD56 expression in MDS was well established from many previous studies, we included bone marrows from a series of MDS cases for comparison. Forty cases from negative staging bone marrow for lymphoma were used as negative controls. We showed that aberrant CD56 expression on granulocytes and monocytes can be seen in all subtypes of MPN. Using a cutoff value of 10 %, we found aberrant CD56 expression on granulocytes in 15 % CML, 10 % ET, 13 % PV, and 37 % PMF. CD56 was negative (<2 %) on granulocytes and monocytes in all 40 cases of negative control bone marrow. In high-grade MDS, as expected, CD56 was positive on granulocytes in 18 % cases and on monocytes in 38 % cases. The frequencies are in agreement with the reported frequencies from previous studies [2, 14].
Lanza et al. reported higher frequencies of CD56 expression on granulocytes (approximately 50 %) in all four types of MPN using Leu19 (My31) clone. Using a second CD56 clone (Eric 1), the authors found similar frequencies in CML and PMF, but much lower frequencies in PV and ET (5 and 15 %, respectively) [4]. In our series, CD56 expression was detected on granulocytes in 15 % CML. It is unclear why higher frequency was observed in Lanza's study. It may be attributed to different CD56 clones, instruments, and/or analysis software used in their study. The study also observed a higher frequency of CD56 expression in PMF; however, the results may not be completely reliable due to an additional factor that low numbers of PMF cases were studied (four cases) [4]. A more recent study by Feng et al. in a large series of PMF reported positive CD56 expression on granulocytes in 34 % cases (using the same cutoff criteria as we used), which is in agreement with our results [5]. In ET and PV, we observed relatively lower frequencies and fluorescence intensity of CD56 expression than in PMF. This may reflect the different biologic behavior of these diseases in which ET and PV primarily affect platelets and erythroid cells rather than granulocytes. However, the fact that CD56 expression can be seen in all subtypes of MPN supports the concept that MPN is a stem cell disorder that affects all hematopoietic lineages.
CD56 expression may not be completely specific for neoplastic clones in MPN. Previous reports have suggested that chemotherapy or bone marrow transplantation may induce abnormal CD56 expression on normal recovering granulocytes [13]. CD56 expression on granulocytes might also be seen in individuals receiving growth factor treatment [13]. We analyzed CD56 expression on granulocytes in normal bone marrows from patients who previously received chemotherapy (27 patients) or bone marrow transplantation (47 patients). One patient from post-chemotherapy bone marrow and one patient from post-transplantation bone marrow showed greater than 10 % CD56-positive granulocytes. In addition, approximately one third of patients from both groups showed between 2 and 10 % CD56-positive granulocytes (data not shown). Overall, the percentage and intensity of CD56-positive granulocytes were generally lower than those found in MPN and MDS. None of the cases showed greater than 20 %. These results indicate that detection of higher percentage and/or higher fluorescence intensity of CD56-positive granulocytes is more likely indicative of abnormal clones. The mechanism of the increased CD56 expression on granulocytes in these patients is not completely clear. This may represent a change of antigenic pattern when granulocytes undergo rapid regeneration, as many of the patients were under the treatment of growth factors. A study by Finnin et al. on the effect of granulocyte and monocyte colony stimulating factor showed that growth factors might cause certain antigenic modification in monocytes. The antigen changes were likely from a subpopulation of more immature monocytes. The study did not, however, found increase of CD56 expression on monocytes. Neither were granulocytes evaluated in the study [15]. In our series, 46 % post-chemotherapy cases and 15 % post-BMT cases showed greater than 10 % CD56-positive monocytes, many with higher percentage of CD56-positive cells (mean 21 % in both groups). Whereas granulocytes demonstrated much lower frequency and percentages of CD56 expression in both groups, with only two patients (of 74) had greater than 10 % CD56-positive granulocytes. One patient received chemotherapy for B cell lymphoblastic leukemia and the second patient received bone marrow transplantation for high-grade MDS. However, neither patient had received growth factor treatment immediate before the bone marrow biopsy. Interestingly, at the time of the bone marrow biopsies, both patients had experienced episodes of neutropenic fever and were treated with antibiotics. Whether neutropenic fever can induce CD56 expression on granulocytes is in question. Rather, the expression of CD56 in these patients is likely a nonspecific reactive change rather than expansion of abnormal clones. These results indicate that upregulation of CD56 is more likely be seen in monocytes in posttreatment bone marrow. Further study to follow-up these patients to monitor the trend of CD56 in relation with disease status is necessary to better understand the effect of therapy on CD56 expression.
Since increased CD56 expression can be seen in large percentages of monocytes from normal bone marrows after chemotherapy and bone marrow transplantation, it appears that CD56 expression in granulocytes is more specific in defining leukemia-associated populations in these patients. Other factors may also affect the accuracy of assessing CD56 expression on monocytes in MPN. Patients with MPN often present with erythrocytosis, granulocytosis, and/or thrombocytosis, with monocytopenia. Often, no discrete monocyte population can be delineated on CD45/side scatter histogram. In our series of MPN cases, monocytes were often present at less than 5 % without forming a discrete population on CD45/side scatter histogram (data not shown). Gating of monocytes simply by using CD45/side scatter approach may be difficult to isolate a pure population. Monocytes can be more accurately selected using a monocyte-specific antigen such as CD14. However, this approach may not always be available in clinical laboratories due to the antibody panels selected in each laboratory, in which CD56 is not always paired in the same tube with CD14. These factors further limit the utility of analyzing CD56 on monocytes in MPN in routine clinical flow cytometry laboratory practice.
Certain myelodysplastic/myeloproliferative neoplasms, such as chronic myelomonocytic leukemia (CMML), may enter into the differential diagnoses with MPN and reactive monocytosis. Previous studies had shown that high frequency of CD56 expression was present in CMML monocytes, but it was not specific to CMML as CD56 may also express on monocytes in conditions of reactive monocytosis [16]. Further study of CD56 on granulocytic components in CMML and reactive monocytosis may have value in differential diagnoses of these entities.
To further prove that CD56-positive granulocytes represent abnormal clones, we analyzed patients who had multiple consecutive specimens and compared CD56 expression with corresponding bone marrow morphology and genetic data. Our results showed correlation of CD56 expression with bone marrow morphologic and molecular evidences of abnormal clones in all five patients (six series of specimens). CD56 expression paralleled with either the quantity of BCR/ABL1 transcripts by quantitative PCR, percentage of bone marrow recipient cells by bone marrow engraftment study, or bone marrow morphology. In the two cases of CML, the percentages of CD56-positive granulocytes decreased in parallel with the reduction of the quantity of BCR/ABL1 transcripts. Interestingly, when BCR/ABL1 turned positive following a previous negative result, CD56-positive granulocytes reemerged as well (Fig. 2a). Similar results were observed following bone marrow transplantation. In the two cases of PMF studied, CD56-positive granulocytes were detected when residual diseases were evident by bone marrow morphology, while CD56 granulocytes were not detected when bone marrow morphology normalized. These results further support the notion that CD56-positive granulocytes in MPN represent abnormal clones and monitoring CD56 abnormal granulocytes in serial bone marrow samples may have a value in follow-up of treatment.
Clinically, distinguishing PMF from ET may often be diagnostically challenging especially when PMF is presented in pre-fibrotic stage. Statistical analyses of the CD56 expression frequencies in these two categories showed significant difference in CD56 expression on granulocytes (p = 0.036), but not on monocytes (p > 0.05). This finding suggests that CD56 expression on granulocytes may have a value in the differential diagnoses of PMF and ET. However, our series did not include cases of PMF in pre-fibrotic stage, which are known to pose the greatest overlaps with ET. It would be interesting to further collect cases of pre-fibrotic PMF and compare the frequency of CD56 expression with the reported cohort in our series.
In summary, analysis of aberrant CD56 expression on granulocytes is a useful adjunct in evaluation of suspected MPN. CD56 expression can be seen in all subtypes of MPN, with higher frequency in PMF. CD56-positive granulocytes likely represent neoplastic population as it is closely associated with the amount of abnormal clones by molecular evidence. Therefore, CD56 may have a value in both initial diagnosis and monitor disease course following treatment in MPN. In clinical practice, since flow cytometry result is usually available before complete morphologic and genetic information, detection of CD56-positive granulocytes may raise initial suspicion of a neoplastic process, and hence prompting further evaluation. However, it should be emphasized that CD56 expression is not specific to MPN and should only be used in conjunction with other ancillary and confirmatory tests in establishing the diagnosis. Caution should be taken when assessing CD56 in patients following chemotherapy or bone marrow transplantation as a small percentage of MPN may have low levels of CD56 expression on granulocytes. Higher frequencies of CD56 expression were detected on monocytes, but assessing monocytes is less useful due to considerably fewer, often insufficient numbers of monocytes in MPN and its low specificity for abnormal clones. Future study of CD56 expression by immunohistochemistry on formalin-fixed bone marrow tissue sections may prove to be more valuable since flow cytometry is not always performed in all the MPN patients. In addition, further study of molecular profile on purified CD56-positive and CD56-negative granulocytes will be useful to better understand the biology of CD56 overexpression in MPN.
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, PIleri SA, Stein H, Thiele J, Vardman JW (2008) World Health Organization of classification of haematolymphoid malignancies, 4th edn. International Agency for Research in Cancer, Lyon
Stetler-Stevenson M, Arthur DC, Jabbour N, Xie XY, Molldrem J, Barrett AJ, Venzon D, Rick ME (2001) Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood 98(4):979–987
Westers TM, Ireland R, Kern W, Alhan C, Balleisen JS, Bettelheim P, Burbury K, Cullen M, Cutler JA, Della Porta MG, Drager AM, Feuillard J, Font P, Germing U, Haase D, Johansson U, Kordasti S, Loken MR, Malcovati L, te Marvelde JG, Matarraz S, Milne T, Moshaver B, Mufti GJ, Ogata K, Orfao A, Porwit A, Psarra K, Richards SJ, Subira D, Tindell V, Vallespi T, Valent P, van der Velden VH, de Witte TM, Wells DA, Zettl F, Bene MC, van de Loosdrecht AA (2012) Standardization of flow cytometry in myelodysplastic syndromes: a report from an international consortium and the European LeukemiaNet Working Group. Leukemia 26(7):1730–1741
Lanza F, Bi S, Castoldi G, Goldman JM (1993) Abnormal expression of N-CAM (CD56) adhesion molecule on myeloid and progenitor cells from chronic myeloid leukemia. Leukemia 7(10):1570–1575
Feng B, Verstovsek S, Jorgensen JL, Lin P (2010) Aberrant myeloid maturation identified by flow cytometry in primary myelofibrosis. Am J Clin Pathol 133(2):314–320
Raspadori D, Damiani D, Lenoci M, Rondelli D, Testoni N, Nardi G, Sestigiani C, Mariotti C, Birtolo S, Tozzi M, Lauria F (2001) CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia 15(8):1161–1164
Ely SA, Knowles DM (2002) Expression of CD56/neural cell adhesion molecule correlates with the presence of lytic bone lesions in multiple myeloma and distinguishes myeloma from monoclonal gammopathy of undetermined significance and lymphomas with plasmacytoid differentiation. Am J Pathol 160(4):1293–1299
Mann KP, DeCastro CM, Liu J, Moore JO, Bigner SH, Traweek ST (1997) Neural cell adhesion molecule (CD56)-positive acute myelogenous leukemia and myelodysplastic and myeloproliferative syndromes. Am J Clin Pathol 107(6):653–660
Harrington A, Olteanu H, Kroft S (2010) The specificity of immunophenotypic alterations in blasts in nonacute myeloid disorders. Am J Clin Pathol 134(5):749–761
Dunphy CH (2011) Comparative analysis of detecting monocytic cells and their aberrancy. Appl Immunohistochem Mol Morphol 19(4):336–340
Kern W, Bacher U, Haferlach C, Schnittger S, Haferlach T (2011) Acute monoblastic/monocytic leukemia and chronic myelomonocytic leukemia share common immunophenotypic features but differ in the extent of aberrantly expressed antigens and amount of granulocytic cells. Leuk Lymphoma 52(1):92–100
Lacronique-Gazaille C, Chaury MP, Le Guyader A, Faucher JL, Bordessoule D, Feuillard J (2007) A simple method for detection of major phenotypic abnormalities in myelodysplastic syndromes: expression of CD56 in CMML. Haematologica 92(6):859–860
Loken MR, van de Loosdrecht A, Ogata K, Orfao A, Wells DA (2008) Flow cytometry in myelodysplastic syndromes: report from a working conference. Leuk Res 32(1):5–17
van de Loosdrecht AA, Westers TM, Westra AH, Drager AM, van der Velden VH, Ossenkoppele GJ (2008) Identification of distinct prognostic subgroups in low- and intermediate-1-risk myelodysplastic syndromes by flow cytometry. Blood 111(3):1067–1077
Finnin M, Hamilton JA, Moss ST (1999) Characterization of a CSF-induced proliferating subpopulation of human peripheral blood monocytes by surface marker expression and cytokine production. J Leukoc Biol 66(6):953–960
Xu Y, McKenna RW, Karandikar NJ, Pildain AJ, Kroft SH (2005) Flow cytometry analysis of monocytes as a tool for distinguishing chronic myelomonocytic leukemia from reactive monocytosis. Am J Clin Pathol 124:799–806
Acknowledgments
The authors wish to thank Linda Blumstein, Kathleen Schroeder, and Liam Nisenfeld for technical support and Endi Wang, MD for review of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, P., Metrebian, F., Dulau-Florea, A. et al. Aberrant expression of CD56 on granulocytes and monocytes in myeloproliferative neoplasm. J Hematopathol 6, 127–134 (2013). https://doi.org/10.1007/s12308-013-0190-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-013-0190-z