Abstract
Systemic acquired resistance protects plants against a broad spectrum of secondary infections by pathogens. A crucial compound involved in the systemic spread of the threat information after primary pathogen infection is the C9 oxylipin azelaic acid (AZA), a breakdown product of unsaturated C18 fatty acids. AZA is generated during lipid peroxidation in the plastids and accumulates in response to various abiotic and biotic stresses. AZA stimulates the expression of AZELAIC ACID INDUCED1 (AZI1), and a pool of AZI1 accumulates in the plastid envelope in association with AZA. AZA and AZI1 utilize the symplastic pathway to travel through the plasmodesmata to neighbouring cells to induce systemic stress resistance responses in distal tissues. Here, we describe the synthesis, travel and function of AZA and AZI1 and discuss open questions of signal initiation and propagation.
Similar content being viewed by others
Metabolites and proteins involved in systemic resistance
Local infections by pathogens cause resistance in distal tissues and protects them against subsequent pathogen attacks. The resistance in the whole plant is known as systemic acquired resistance (SAR) (Vlot et al. 2021). The resistance in the distal tissue requires mobile signal molecules which are generated at the local infection site and transported to uninfected distal tissues either through the plant body itself or via the air as volatiles (Kachroo and Kachroo 2020; Shine et al. 2019). Several signaling molecules which are at least partially transported through apoplastic or symplastic compartments or the phloem have been identified: this includes the lipid-derived oxylipin azelaic acid (AzA), glycerol-3-phosphate, pipecolic acid, N-hydroxy-pipecolic acid, dehydroabietinal, nitric oxide and reactive oxygen species (ROS) (Kim and Lim 2023; Gao et al. 2021, 2014; Siebers et al. 2016; Hartmann et al. 2017, 2018; Shine et al. 2019; Dempsey and Klessig 2012). The volatile monoterpenes α- and β-pinene and methyl salicylate is spread through the air to distal parts of the same plant but can also be perceived by neighbouring plants (Riedlmeier et al. 2017; Gong et al. 2023). Transport and function of these traveling signaling molecules are imbedded into a complex network with multiple interactions which included the proteins DEFECTIVE IN INDUCED RESISTANCE 1 (DIR1) and AZELAIC ACID INDUCED 1 (AZI1) (Yu et al. 2013). Here, we focus on the origin, transport and signalling of AZA and its interaction with AZI1. The involvement of AZA in whole plant immunity or signaling has been mainly studied in Arabidopsis infected with Pseudomonas syringae pv tomato (Pst) (Miranda de la Torre 2023; Banday et al. 2022; Witteck et al. 2014; Wang et al. 2014; El-Shetehy et al. 2015; Lim et al. 2016; Cecchini et al. 2019, Jung et al. 2009; Yu et al. 2013; Zoeller et al. 2012). AZA accumulation lowers the infection and disease spread also in systemic tissue of tomato, soybean (Korenblum et al. 2020) and crops (Saikia et al. 2020). The requirement of AZI1 for AZA function has been often demonstrated with azi1 knock-out lines: all studies demonstrate that endogenous AZA or exogenously applied AZA requires AZI1 for systemic resistance (Gao et al. 2021; Dutton et al. 2019; Shine et al. 2019; Wang et al. 2016; Cecchini et al. 2015, 2019; Yu et al. 2013; Jung et al. 2009).
AZA and its generation in plastids
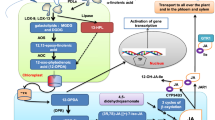
AZA is a saturated linear C9 dicarboxylic acid (HOOC(CH2)7COOH) with multiple and diverse functions in eukaryotic organisms. In plants, AZA is mainly found in the plastid and plastid envelope (Pitzschke et al. 2014a), where it accumulates as a marker for lipid peroxidation under biotic and abiotic stress conditions (Adám et al. 2022). The plastid membranes contain lipid galactosides such as oleic acid, linolenic acid, or linoleic acid. These 18 carbon fatty acids contain a double bond at C9 which is hydrolysed to generate an AZA molecule (Zoeller et al. 2012; Yu et al. 2013; Wong et al. 2006; Gao et al. 2014). Zoeller et al. (2012) showed that three oxidation mechanisms are involved in AZA formation.
Singlet oxygen (1O2) is the major cause for lipid peroxidation and thus AZA formation under unstressed conditions dominates in green tissue (Triantaphylides et al. 2008; Triantaphylides and Havaux 2009; Farmer and Mueller 2013). In plastids, the major source of the nonradical electrophilic 1O2 is generated as a by-product during light capture at photosystem II, when energy is transferred from an excited chlorophyll molecule to ground-state O2 (Halliwell 2006; Kim and Apel 2013; Pospíšil 2016). 1O2 and other reactive oxygen species such as hydrogen peroxide and hydroxyl radical also arise during the interaction of light with chlorophyll precursors, i.e., protochlorophyllide or protoporphyrin IX, in the presence of molecular oxygen (Tripathy and Oelmüller 2012; Ryter and Tyrrell 1998; Fujii 2023). Photosynthetic organisms have developped quenching mechanisms to restrict 1O2 accumulation, e.g., via carotenoids, which convert 1O2 to ground-state O2, but under high light conditions, some 1O2 accumulates in or in the vicinity of the thylakoid membranes. In a non-catalytic reaction, one 1O2 generates one lipid hydroperoxide in the thylakoid membrane (Farmer and Mueller 2013).
Enzymatic lipid peroxidation is initiated by lipoxygenases (LOXs). LOX oxidize free fatty acids in the cytosol or chloroplasts, catalyze the hydroperoxidation of C-18 unsaturated fatty acids, thereby initiating several oxylipin pathways including the jasmonate and hydroperoxide lyase pathway (Mosblech et al. 2009) which results in the synthesis of intermediates for several defense-related products, including AZA and the jasmonic acid (Vick and Zimmermann 1987; Rosahl et al. 2005). In case of linolenic acid, the enzyme catalyzes the stereospecific oxygenation of the position 13 of linolenic acid to form linolenic acid 13-hydroperoxide. Relevant for AZA biosynthesis is the plastid localized LOX2, which participates in lipid peroxidation, but knock-out lines demonstrate that this enzyme is not essential for AZA biosynthesis, at least after pathogen attack (Zöller et al. 2012). LOX expression is developmentally controlled and stimulated in response to wounding, pathogen attack and water deficit (Bell and Mullet 1991, 1993; Siedow 1991; Koch et al. 1992; Ohta et al. 1991). The enzyme is involved in many biotic stress responses including priming processes (Losvik et al. 2017; Rustgi et al. 2019; Zhao et al. 2023), as well as drought, cold and salt stress (cf. De Domenico et al. 2012; Nieto-Garibay et al. 2022; Liu et al. 2017; Du et al. 2013; Shi et al. 2022). Finally, hydroperoxide lyases catalyse the cleavage of C–C bonds in the hydroperoxides to generate oxylipins, including AZA (Matsui et al. 2006).
Radical-catalyzed lipid peroxidation is mainly initiated by the the radical source H2O2. In the presence of irons, H2O2 is degraded to hydroxyl radicals (HO•) and superoxide anion radicals (OO•−) (Halliwell 2006; Mittler et al. 2004). HO•, but not OO•−, abstracts hydrogen from fatty acids, which generates various lipid peroxides in the presece of oxygen. The free radicals break down 18:1, 18:2 and 18:3 fatty acids. These processes can also be catalyzed by other radicals, such as reactive nitrogen species. Peroxynitrite (•ONOO), the reaction product of nitric oxide (NO) and OO•− (Vandelle and Delledome 2011) reacts with CO2 to generate carbonate radicals (Radi 1998). NO triggers synthesis of various ROS species (superoxides, hydroxyls, 1O2 or H2O2) and all radicals act additively to catalyze the oxidation of free C18 unsaturated fatty acids to generate AZA (Wendehenne et al. 2014; cf. also Yu et al. 2013). Different ROS species are also generated during abiotic stress (Li and Kim 2021; and ref. therein) indicating that both biotic and abiotic stress may contribute to AZA production via free radicals.
The pathways do not operate completely independent of each other. For instance, during pathogenesis, photosystem II activity is normally inhibited with results in an increased accumulatioin of 1O2 (Triantaphylides et al. 2008; Triantaphylides and Havaux 2009). Furthermore, 1O2-mediated lipid fragmentations generate radicals, which cause additional membrane and lipid damage. Zoeller et al. (2012) demonstrated that the free radical-catalyzed galactolipid fragmentation mechanism is mainly responsible for AZA formation in Arabidopsis after pathogen (Pseudomonas syringae pv tomato DC3000) infection. Therefore, besides functioning a mobile defense signals for whole plant immunity, AZA is a marker for lipid oxidation (cf. Cecchini et al. 2019; Shine et al. 2019; Gao et al. 2021).
AZI1 and AZA transport
The transport of AZA or the AZA signal requires the lipid transfer protein AZI1, a member of the hybrid proline-rich protein (HyPRP) family. A pool of AZI1/EARLY ARABIDOPIS ALUMINIUM INDUCED1 (EARLI1), a close paralog of AZI1, localizes to the plastid envelope. Mainly based on experiments where AZA was exogenously applied to leaf tissue, it was shown that AZA induces AZI1 and EARLI1 expression in the nucleus (Jung et al. 2009).
AZI1 contains an amino terminal hydrophobic domain (a signal peptide (cf. below)) which is followed by the proline-rich region. These two domains (signal peptide and proline-rich region) can be considered as a non-cleavable bipartite N-terminal signature that shares features with plastid transit peptides, and the transmembrane domain of the signal peptide which anchors the protein to membranes (Fig. 1). The transmembrane domain is required for the ring‐like pattern of plastid membrane association, i.e., AZI1´s association with the plastid envelope. Cecchini et al. (2021) showed that the signal peptide/hydrophobic domain and the proline-rich region are required for targeting of AZI1 to the plastid outer envelope membrane. AZI1´s paralog EARLI show the same protein structure and targeting features. Targeting of AZI1 and EARLI1 to chloroplasts is increased during SAR (Cecchini et al. 2021). Application of flg22 (a 22 amino acids-long peptide from the bacterial flagellin that functions as pathogen associated molecular pattern) results in elevated AZI1/EARLI1 protein levels and promotes their protein pools in the plastid fraction. Also the defense-associated MITOGEN-ASSOCIATED PROTEIN KINASE3 (MAPK3) and -6 are involved in promoting the accumulation of AZI1 at plastids (Cecchini et al. 2021). MAPK3 and -6 can phosphorylate AZI1 in vitro and in vivo (Pitzschke et al. 2014a; Cecchini et al. 2021) (Fig. 1), which promotes entry of AZI1 into and sorting at the organelle, in particular under stress (cf. below) (Cecchini et al. 2021). Stress induces AZA production and AZI1 could facilitate the movement of AZA, and potentially also other plastid oxylipins related to stress resistance from the plastid outer membrane system to the endoplasmatic reticulum (Cecchini et al. 2021). The plastid outer membrane functions as a defense platform against several biotic and abiotic stresses, since it contains enzymes for the synthesis of fatty acids and for a variety of fatty acid derivatives (Breuers et al. 2011). Digalactosyldiacylglycerol synthase for fatty acid biosynthesis (Froehlich et al. 2001a) and enzymes for the breakdown of fatty acid hydroperoxides are located in the chloroplast envelope membranes (Blée and Joyard 1996; Froehlich et al. 2001b). For instance, the hydroperoxide lyase is an outer envelope membrane enzyme that catalyzes the first step towards defense-related aldehydes (Blée and Joyard 1996; Howe and Schilmiller 2002; Kishimoto et al. 2008). AZA might be generated at the envelope membrane or transported to it from the thylakoid membrane. The radical precursor H2O2, which is the major source for AZA formation after pathogen attack (Zoeller et al. 2012), is generated by the NADH oxidase at the apoplastic side of the plasma membrane after pathogen attack and might have better or faster access to the outer envelope membrane than to the thylakoid membranes, because diffusion of H2O2 across membranes (plasma membrane alone or plasma membrane and inner envelope membrane of chloroplasts) is limited (Bienert et al. 2006). Transport of AZA from the thylakoid membrane to the envelope membrane requires passage through the inner envelope membrane, a process that most likely involves a transport molecule. The dynamic non-covalent interaction between the plastid outer membrane and the endomembrane system play important roles in lipid trafficking and trafficking of membrane-bound signalling molecules (Breuers et al. 2011). This allows AZA movement within the cell membranes and translocation to neighbouring cell via plasmodesmata (cf. below). How AZI1 supports AZA trafficking is not clear.
AZI1 consists of secretion signal (yellow), a proline-rich domain (PRD, green), and a characteristic eight-cysteine-motif (8-CM) segment (blue) (cf. Jose-Estanyol et al. 2004). The secretion signal and the PRD also function as a bipartite non-cleavable N-terminal transit sequence for plastid import that harbors a transmembrane domain and anchors the protein to membranes. The cysteines in the 8-CM segment are underlined and bold. Five putative MAPK phosphorylation sites (serine, S; threonine, T) are in red (Pitzschke et al. 2016). The putative MAPK interaction sites with the consensus (R/K x2–6 L/IxL/I) is in purple (Cecchini et al. 2021). The hydrophobic C-terminal 8-CM domain is also present in lipid transfer proteins, amylase inhibitors and 2S albumins (Dvorakova et al. 2007; Jose-Estanyol et al. 2004)
Gel retardation assays showed that AZI1 is posttranslationally modified. AZI1 forms protein complexes and MAPK3 (Pitzschke et al. 2014b) and the potential phosphorylation sites in AZI1 are located in the proline-rich region (Fig. 1). Pitzschke et al (2014b) demonstrated that phosphorylation is physiologically relevant. The azi1 mutant is hypersensitive to salt stress, while AZI1-overexpressor lines are more tolerant than the wild-type. Since AZI1 overexpression in the mapk3 background partially alleviates the salt-hypersensitive phenotype MAPK3 which further involved in the AZI1-conferred robustness against this stress. Furthermore, the proline-rich region found in HyPRPs shows similarities to arabinogalactan proteins which are modified by proline hydroxylation and subsequent O-glycosylation. This posttranslational modification occurs also in AZI1 since inhibition of prolyl hydroxylase reduced the apparent protein size of AZI1 (Pitzschke et al. 2016). These protein modifications are stress-independent and unrelated to the phosphorylation by MAPKs. It remains to be determined whether this modification affects other physiological processes, or AZI1 sorting in the cell.
Zöller et al. (2012) showed that local AZA production was not compromised in the azi1 mutant, suggesting that AZA accumulation is independent of AZI1. Other family members have not yet been tested. Likewise, whether AZI1 accumulation at the plastids is affected by AZA, has also not yet been investigated. This also holds true for the AZI1´s paralog EARLI1. Like AZI1, EARLI1 accumulates at the plastid envelope during defense, and the regulation of the expression of the EARLI1 gene exhibits similarities to that of AZI1. Furthermore, EARLI1 is also involved in systemic defense priming and SAR (Cecchini et al. 2021).
AZI1 is a member of the HyPRP superfamily, with 28 members in Arabidopsis. All HyPRPs have a transmembrane domain, a proline-rich region, and a lipid transfer protein domain (a characteristic eight-cysteine-motif segment; cf. Jose-Estanyol et al. 2004) (Fig. 1). The precise subcellular location(s) and function(s) for most HyPRP family members are unknown (Banday et al. 2022). Besides AZI1, also HyPRP members have a pool of proteins that target plastid outer envelope membranes via their proline-rich domains. Two HyPRPs are associated with thylakoid membranes (Banday et al. 2022), and AZI-LIKE2 (AZL)2, AZL13, AZL14 and ELP (EXTENSIN-LIKE PROTEIN) are outer envelope membrane proteins. AZL3 and DRN1 (DISEASE RELATED NONSPECIFIC LIPID TRANSFER PROTEIN1) are either thylakoid or/and envelope membrane proteins. Most of the plastid- and nonplastid-localized family members also have pools that are localized to the endoplasmic reticulum, plasma membrane, or plasmodesmata (Banday et al. 2022). Some of the HyPRPs are cell-wall structural proteins, and they are either positive or negative regulators of abiotic and biotic stress responses in different plant species (Saikia et al. 2020). In crop plants, they participate in cold, drought, salt and oxidative stress responses, down-regulate ROS scavenging genes or participate in basal defense against pathogens (cf. Table 1 in Saikia et al. 2020). Besides its plastid/envelope membrane localisation, also part of AZI1 is found in the apoplast (Pitzschke et al. 2016). The N-terminal secretion signal directs an AZI1-fluorescent protein fusions to the endoplasmatic reticulum (Yu et al. 2013) and the fusion protein is further secreted via the secretory pathway to the cell surface (Pitzschke et al. 2014b; Zhang and Schlappi 2007). Furthermore, proteomic studies identified AZI1 in plasmodesmata and the plasma membrane (Fernandez-Calvino et al. 2011; Mitra et al. 2007, 2009). Pitzschke et al. (2016) also showed that a proportion of AZI1 is secreted by protoplasts, however, the majority of the protein remained in the protoplast fraction. This demonstrates similarities between AZI1 and apoplastic HyPRPs with stress-regulatory functions, and suggests that translocation of AZI1 or an AZI1-derived mobile signal in the establishment of SAR could also occur via the apoplastic space, although to a lesser extent than the plastid-originated signaling pathway. Two systemic signaling pathways, one starting in or at plastids and another one from secreted AZI1, exists, has to be investigated. Moreover, the role of AZA in a scenario with secreted AZI1 is not clear; it is conceivable that AZA only induces AZI1 expression to promote the apoplastic AZI1 pool. Furthermore, the unique function of AZI1 among the HyPRPs is not well understood. Although knock-out mutants showed that AZI1 cannot be replaced by other HyPRPs including EARL1, it has not yet been investigated in details whether (or to what extent) other HyPRPs (except EARLI1, cf. Cecchini et al. 2021) can induce systemic signalling.
AZA (signal) movement to systemic tissue and priming
Threat information travels from the local site exposed to a threat stimulus to distal tissues, which somehow stores the threat information, a process called priming. In primed tissues, the defense responses are not activated yet. However, if primed tissue is exposed to the same or similar threat, that induced the primed state, the response differs from that of the unprimed tissue, since it activates induced systemic resistance or SAR programs (Conrath et al. 2015; Fu and Dong 2013; Oelmüller 2021). Defense priming mediated the AZA/AZI1 results in a faster and stronger activation of defense and antioxidant genes, genes for phytohormones and enzymes involved in the synthesis of defense-related metabolites including volatiles. In tobacco cells, genes for pathogenesis-related proteins and enzymes involved in phenylpropanoid pathway and chlorogenic acid metabolism as well as signal transduction components responded to AZA application in systemic tissue (Djami-Tchatchou et al. 2017). Defense related metabolites included caffeoylputrescine glucoside and related secondary compounds. Salicylic acid is the major phytohormone activated upon AZA application. This raises the question how AZA induces a primed state. Since AZA or the AZA-derived signal does not directly activate defense genes, they should target molecules which alter the physiological state in the primed cell. A possible scenario has been recently described by Miranda de la Torre et al. (2023). Priming involves chromatin modifications for a faster/stronger activation of defense genes (Miranda de la Torre et al. 2023). The chromatin regulator MORPHEUS MOLECULE1 (MOM1) functions as a priming factor which affects the expression of several immune receptor genes. AZA treatments reduce MOM1 expression in systemic tissues and lower MOM1 levels sensitize the primed tissue to biotic stresses. In plants exposed to stressful conditions, the decrease in MOM1 facilitates the upregulation of immune receptors, which improves the perception of future attacking pathogens and the amplification of the plant defense responses. Therefore, MOM1 is as a chromatin factor that negatively regulates the defense priming induced by AZA.
Besides its involvement in defense priming, AZI1 is also required for the reduction of stomata density to restrict Pseudomonas entry, as shown by Dutton et al. (2019). This suggests that–besides relatively fast immune priming in distant tissue—AZA also participates in long-term developmental programs.
Translocation of the AZA-dependent information from local to distal tissue can occur in three ways. The compounds either travel directly from the local application site to the distal tissue, or activates traveling of other signalling compounds, or induces signalling events that activate its own de novo synthesis along the traveling path and ultimately in the distal tissue (Hartmann et al. 2018; Wang et al. 2018; Cecchini et al. 2019; Vlot et al. 2021). Since AZA is generated by lipid peroxidation, de novo synthesis along the traveling path or in distal tissue requires cells where lipid oxidation occurs. Jung et al. (2009) were among the first who proposed that AZA is transported to non-infected systemic leaves after P. syringae infection. However, Zoeller et al. (2012) showed that the AZA level in the systemic leaves 24 h after local infection was not elevated in comparison to the levels found in non-infected control leaves. Furthermore, AZA-inducible AZI1 expression was not stimulated in the systemic leaves.
AZA is found in roots and leaves, and Cecchini et al. (2015) showed that exogenously applied 14C-AZA can move within the plant body. Movement of label from one leaf (the application site) to total systemic tissues (aerial stem/leaves and roots) was significantly reduced in azi1 and earli1-1 compared to wild-type plants (Cecchini et al. 2015). In wild-type, a large amount of the signal that moved within aerial tissues was detected in very young leaves. Interestingly, a lot of the label also moved systemically from leaves to the roots. The azi1 and earli1 mutants showed significant decreases in label uptake into leaf discs (∼25%) compared to the wild-type, when AZA was applied exogenously. The authors concluded that movement and uptake of AZA (and possibly AZA derivatives) partially depends on AZI1 and EARLI1. However, Cecchini et al. (2019) showed that deuterium-labeled AZA applied to the roots does not move to aerial tissues, although AZA application to roots triggers systemic immunity in leaves. This suggests that AZA can travel root-, but not shootwards. The authors postulated an AZI1/EARLI1/MAPK3/-6-dependent pathway and the AZA effects may involve additional mobile signals. Apparently, translocation of exogenously applied AZA depends on the directions and tissue. Since AZA-induced immune responses in distal tissues are not always associated with its translocation to this tissue or with elevated AZA levels in this tissue, the involvement of additional molecules is likely. Furthermore, whether AZA travels alone or in association with AZI1, remains to be investigated.
Direct or indirect interactions of AZA with other systemic signaling molecules involved in SAR responses have been reported which might be involved in the translocation of the information to distal tissue. Besides EARLI1 and the above mentioned pipecolic acid, N-hydroxy-pipecolic acid, dehydroabietinal, glycerol-3-phosphate, the monoterpenes α- and β-pinene, methyl salicylate, NAD(P) and DIR1, the hormone salicylic acid, the free radicals NO and ROS have been described (Gao et al. 2021; Huang et al. 2023; Riedlmeier et al. 2017; Shine et al. 2019; Yu et al. 2013; Dempsey and Klessig 2012; El-Shetehy et al. 2015; Gao et al. 2015). Salicylic acid acts in parallel with the two radical signals NO and ROS, and simultaneous activation of the salicylic acid and NO/ROS pathway is essential for full SAR responses. NO/ROS acts upstream of AZA, as well as glycerol-3-phosphate (Wang et al. 2014). Yu et al. (2013) demonstrated that a feedback regulatory loop between glycerol-3-phosphate and the lipid transfer protein DIR1 and AZI1 mediates AZA-induced systemic immunity. Wang et al. (2016) showed that soluble carbohydrates might function as signal substances in the systemic immunity of Arabidopsis. The expression of the sugar signaling genes (SUS1, -2, -3, -6, SUT1, HXK1, -2, SNRK1.1, -1.2, -1.3, ERD6, TPS1, TOR and bZIP11) in local and distal leaves after infection of avirulent P. syringae was changed in plants with modulated AZI1 activities (knock out and overexpressor lines), indicating that sugar-related genes are involved in regulation of the systemic immunity mediated by AZI1. This suggests an extended cross-talk between systemic signalling molecules and the primary sugar metabolism, raising the question how AZI1 is integrated into the network.
Besides AZA´s direct participation in defense priming, HyPRPs are also involved in balancing beneficial and pathogenic traits in symbiotic interactions. For instance, HyPRPs regulate the interaction with the plant growth-promoting rhizobacteria Pseudomonas simiae WCS417 in the roots to influence colonization, root system architecture, and/or biomass. Therefore, HyPRPs have broad and distinct roles in immunity, development, and growth responses to microbes and reside at sites that may facilitate signal molecule transport (Banday et al. 2022). Furthermore, some mutants of this family are also affected in both induced systemic resistance and SAR, suggesting overlapping functions with AZI1/EARLI1.
Symplastic transport of AZI1 to the phloem
Movement of small proteins or metabolites to systemic tissues occurs often via the phloem (Dinant and Lemoine 2010). Uploading of AZI1 to the phloem occurs via the symplastic transport (Lim et al. 2016) and the protein reaches the phloem via sorting signals which direct it from the outer plastid membrane to the endoplasmic reticulum and plasmodesmata which transverse the cell wall and join the adjacent cells. Being a lipid-binding and membrane-bound protein, AZI1 appears to travel to the phloem companion cells via direct membrane–membrane contact sites, which have been identified at the outer plastid membrane, the endoplasmatic reticulum and the plasma membrane. In case of lipid transfer proteins such as AZI1 these contact sites also allow exchange of the bound lipids (Breuers et al. 2011; Wang and Benning 2012; Helle et al. 2013, Cecchini et al. 2015). Lim et al. (2016) demonstrated that two plasmodesmata-localized proteins regulated SAR function in both, signalling and plasmodesmata gating of AZI1. While PLASMODESMATA LOCALIZING PROTEIN1 (PDLP1) interacts with AZI1, is required for endoplasmatic reticulum-specific localization of AZI1, and contributes to the intracellular portioning of the protein, PDLP5, which impairs plasmodesmata permeability and thus transport of AZA to the neighboring cell. PDLP1 interacts with PDLP5 which regulates the symplastic transport and plasmodesmata gating (Lee et al. 2011; Lim et al. 2016). PDLP5 knockout mutants increase and overexpressor lines restrict general plasmodesmata permeability (Lee et al. 2011; Wang et al. 2013). Importantly, the pdlp1 mutants contained reduced AZI1-GFP protein levels, although the azi1-gfp transcript levels were not affected (Lim et al. 2016). This suggest that PDLP1 affect the stability of AZI1. Furthermore, in the pdlp1 mutant, AZI1 was primarily localized to the outer plastid membrane, whereas in wild-type plants, the majority of the protein is located in extraplastidic compartments. The studies by Lim et al. (2016) highlight the importance of PDLP1 for AZI1 stability and its traveling from the plastids to the plasmodesmata.
Jung et al. (2009) showed that AZI1 is important for generating vascular sap that confers disease resistance. AZA and petioles exudates failed to induce systemic immunity in azi1 plants. Pathogen-induced exudates from azi1 were inactive when applied to wild-type plants. Therefore, AZI1 modulates production and/or translocation of a mobile signal(s) during SAR. The AZI1 target in the vascular sap is unknown so far.
AZI1 gene activation and abiotic stress
AZI1 expression is stimulated by exogenously applied AZA (Jung et al. 2009). Whether stimulation of the endogenous AZA levels due to lipid peroxidation under stress in planta is a prerequisite for the AZI1 expression is not known.
The best studied biological stimulus for AZI1 activation comes from Arabidopsis leaf infiltration assays with Pseudomonas syringae (cf. Arabidopsis eFP Browser). Similarly effective is the bacterial effector flg22. Late stimulation of AZI1 expression was also observed after co-cultivation of Arabidopsis seedlings with Hyaloperonospora arabidopidis (cf. Arabidopsis eFP Browser). Relatively little is known about the role of AZI1 for other pathogenic or beneficial plant–microbe interactions, including pathogenic fungi, nematodes, insects, mycorrhizal fungi and beneficial endophytes, although some of them can produce AZA. For instance, AZA is produced by P. syringae (Javvadi et al. 2018) or the root colonizing endophytic fungus Piriformospora indica (Kundu et al. 2022), however, whether microbe-synthesized AZA activates AZI1 in plants or participates in defense priming in plants, is not known.
Besides biotic stress, expression profiles demonstrate that the AZI11 mRNA level responds also to abiotic stress, however functional analyses are often missing. Xu et al. (2011) showed that the AZI1 transcript level, as well as that of its paralog EARLI1 (Zhang and Schäppi 2007), increases after exposure of Arabidopsis seedlings to cold. The increase of the AZI1 mRNA level was slow, since more than 6 h at 4 °C was required for the induction. The mRNA level declined to basal levels when the plants were transferred back to room temperatures. Overexpression of AZI1 resulted in reduced electrolyte leakage during freezing damage, while AZI1 knockdown and knockout lines showed increased tendencies in cellular damage after freezing treatment. When Saccharomyces cerevisiae cells were transformed with AZI1 under the control of GAL1 promoter, the survival rate of yeast cells harbouring AZI1 increased after freezing treatment. This demonstrates that AZI1 might be multifunctional and associated with cold tolerance of Arabidopsis (Xu et al. 2011). The involvement of AZI1 in cold stress adaptation is further supported by expression profiling of mutants manipulated in cold stress-acclimation genes. Similar results were obtained for Thelunsiella salsuginea (Wong et al. 2006).
The ICE-CBF-COR (Inducer of CBF Expression—C-repeat Binding Factor—Cold Regulated) signalling pathway is an important regulator for cold-stress acclimation (Gusain et al. 2023). CBF overexpressors show increased cold tolerance and high levels of AZI1 gene expression (Wong et al. 2006). Likewise, DEHYDRATION-RESPONSIVE-ELEMENT-BINDING PROTEIN1 (DREB1) genes are induced by cold stress, and overexpression of DREB1 induced strong expression of other stress-responsive genes, resulting in increased tolerance to high-salt and freezing stresses (Ito et al. 2006). Among the genes which are up-regulated in the DREB1 overexpressor lines after exposure to cold stress is AZI1 (Maruyama et al. 2004).
Besides cold, AZI1 is involved in salinity stress tolerance. Pitzschke et al. (2014b) showed that azi1 mutants are hypersensitive to salt. At 150 mM salt stress, only 7% of the azi1 mutant seeds, 70% of wild-type seeds, and 90% of the seeds of AZI1 overexpressor lines germinated. Furthermore, AZI1 overexpressors thrived better than the azi1 mutants under high salt conditions. Another example provides mutants in with the salt stress signalling gene ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) was manipulated. The overexpressor lines performed better under salt stress and this was associated with the higher expression levels of AZI1 and EARLI1 (Davletova et al. 2005). Furthermore, exposure of the salt tolerant xero-halophyte Haloxylon salicornicum to salt stress resulted lower stearic acid and palmitic acid levels. Panda et al. (2021) speculated that breakage of lipid membranes might lead to higher accumulation of AZA. In conclusion, AZA is also involved in abiotic stress tolerance in various plant species, such as cold (Davletova et al. 2005) and salt tolerance (Atkinson et al. 2013). When Arabidopsis seedlings are exposed to simultaneous biotic and abiotic stresses, AZI1 was down-regulated in leaves and conferred drought susceptibility when overexpressed (Atkinson et al. 2013). More functional analyses are required to understand the role of AZI1 in abiotic stress responses.
Conclusion and open questions
The AZA/AZI1 pathway is involved in both biotic and abiotic stress responses in plants, and a comparative analysis of both stimuli might be helpful to throw more light on the molecular mechanism of systemic resistance. AZA accumulates in response to lipid peroxidation and Zöller et al. (2012) showed that lipid peroxidation is predominantly confined to plastid lipids comprising galactolipid and triacylglyceride species during the interaction of Arabidopsis with P. syringae, i.e. biotic stress. 1O2 was identified as the major cause of lipid oxidation under basal conditions, while LOX2- and free radical-catalyzed lipid oxidation substantially contribute to the increase upon pathogen infection (Zöller et al. 2012). It remains to be determined, whether all AZI1-mediated biotic and abiotic stress responses are linked to AZA and lipid peroxidation in the plastids. Barely anything is known about the role of AZA in AZI1-dependent abiotic stress responses and whether these responses are restricted to local tissues or operate systemically. Systemic signal propagation induced by abiotic stresses might be agriculturally important, e.g. for crop plants with roots in cold soil and aerial parts exposed to extreme heat. Finally, the role of the secreted AZI1 in the apoplast or at the plasma membrane for systemic immune responses and local abiotic stress responses has not yet been studied. This is particularly interesting since other members of the HyPRP family which are found in the apoplast, participate in abiotic stress responses (Saikia et al. 2020).
The initiation of the AZA/AZI1 signaling at plastids needs to be investigated in more details. AZA is present in roots and shoots, but the plastids and the intraorganellar membrane structure as the site of lipid peroxidation differ substantially in two types of plastids. In both organs, AZI1 has been shown to be associated at least in part with plastids. In particular, in the aerial tissue, HyPRPs are mainly found in epidermal cells. Their plastids play key roles in defense against microbes (cf. Banday et al. 2022). Investigating the role of root plastids for the generation of AZA and the AZA/AZI1 interaction is important for unravelling the function of the signalling compounds in roots. Furthermore, AZI1 and EARLI1 expression is strongly down-regulated in roots upon colonisation by fungi (Banday et al. 2022), whereas this was not observed for other HyPRP genes. This raises the question whether AZI1/EARLI1 might have also other functions in roots, e.g., by controlling root colonisation or entry of fungal hyphae into the roots. Banday et al. (2022) have already demonstrated that HyPRPs regulate the interaction with the plant growth-promoting rhizobacteria Pseudomonas simiae WCS417 in the roots to influence colonization, root system architecture, and/or biomass. Further studies are required to understand the differences in the regulation of these HyPRP genes, as well as function and signalling of the proteins in roots and shoots upon pathogenic and beneficial microbial attacks.
Disruption of galactolipids in the plastid membranes by lipid peroxidation generates breakdown products including the oxo-acid AZA which protrude to the aqueous phase. In particular, during membrane repair, this promotes AZA release from the membrane and mobility to neighbouring membranes, either alone or complexed by AZI1. The role of AZI1 for the movement of AZA between membranes requires further attention. Whether membrane disruption during the oxidative process plays a role for AZA movement, should be investigated.
Based on the current knowledge about the regulation of the pathway, localisation of AZI1 in or at plastids and its trafficking to other cellular membranes are early events that proceed the activation of the systemic movement of the priming signal of AZA/AZI1 to distal tissue (cf. Cecchini et al. 2021). Plastid targeting of AZI1 is promoted by MAPK3/6, which is activated by biotic (Pitzschke et al. 2009) and abiotic stresses (e.g. Li et al. 2014). Besides MAPK3/6 activation, biotic and abiotic stresses also generate ROS (Takata et al. 2020; Rodriguez et al. 2010), which–in turn—promote plastid-association of AZI1 via MAPK3/6 signaling, but also lipid peroxidation in plastids which generates AZA. It would be interesting to know how the AZI1 and AZA generation is coordinated (Fig. 2).
A main question centers around the long-distance transportation of AZA or the AZA signal to systemic tissue. If AZA can travel root-, but not shootwards, one has to postulate different mechanisms for the propagation of the information from the roots to the shoots and from the shoots to the roots. AZA is not water soluble and it is well known that membrane contact sites between plastid envelopes, endoplasmatic reticulum, plasma membrane and membrane material at the plasmodesmata are the sites of exchange of small molecules, including AZA (Andersson et al. 2007; Toulmay and Prinz 2011; Li et al. 2020). Small signaling molecules can be rapidly transported to the systemic tissues through the phloem (Gao et al. 2021), however, this is not yet clear for AZA. In addition, the role of AZI1 during the long-distance transport of AZA is not yet understood. If AZA cannot travel shootwards, which signalling compounds are activated to establish SAR in the aerial parts, how are they activated in the roots by AZA/AZI1 and how are they traveling? What is the role of salicylic acid in this scenario?
AZA is only one of the closely related oxylipins, which are generated during lipid peroxidation. Enzymatic oxidative fragmentation of 18:3 lipids results in the accumulation of 9-oxononanoic acid and nonadienal, besides AZA (Zöller et al. 2012; Matsui 2006). The biotin precursor pimelic acid is an important lipid peroxidation product which accumulates during free radical-catalyzed galactolipid fragmentation and its accumulation occurs independently of the LOX2 pathway (Zöller et al. 2012). However, pimelic acid did not induce SAR (Wittek et al. 2014). 9-Hydroperoxy octadecadienoic acid and 9-oxo nonanoic acid can be considered as precursors of AZA and oxidation of exogenously applied 9-oxo nonanoic acid to Arabidopsis establishes SAR, suggesting that it is oxidized to AZA (Wittek et al. 2014). Wittek et al. (2014) showed that—besides AZA—the C9 lipid peroxidation product 9-oxo nonanoic acid is linked to systemic rather than local resistance and the authors suggested that salicylic acid and its upstream regulator ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1) directly or indirectly promotes the accumulation of 9-oxo nonanoic acid, AzA, or one or more of their common precursors possibly by activating one or more pathways that either result in the release of these compounds from galactolipids or promote lipid peroxidation. Furthermore, for several oxylipins, induction of defense responses and root growth inhibition has been reported (Vellosillo et al. 2007; Blée 2002; Prost et al. 2005). Therefore, the role of these lipid peroxidation products needs to be investigated in more details. So far, the AZA/AZI1couple is in the main focus in the field, while the role of EARLI1 and other lipid transfer proteins/HyPRPs in this scenario have been less studied (cf. Banday et al. 2022). As mentioned above, several members of the HyPRP family are involved in stress responses. Whether they can be activated by lipid peroxidation products is not yet known. It is possible that other lipid transfer proteins/HyPRPs have overlapping functions with AZI1, although they are located in different membranes or cellular compartments? It remains to be investigated whether not yet investigated oxylipin/HyPRP combinations may facilitate systemic resistance in response to specific biotic or abiotic stresses.
References
Ádám AL, Kátay G, Künstler A, Király L (2022) Detection of lipid peroxidation-derived free azelaic acid, a biotic stress marker and other dicarboxylic acids in tobacco by reversed-phase HPLC-MS Under non-derivatized conditions. Methods Mol Biol 2526:191–200. https://doi.org/10.1007/978-1-0716-2469-2_14. (PMID: 35657521)
Andersson MX, Goksor M, Sandelius AS (2007) Membrane contact sites: physical attachment between chloroplasts and endoplasmic reticulum revealed by optical manipulation. Plant Signal Behav 2:185–218
Atkinson NJ, Lilley CJ, Urwin PE (2013) Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiol 162(4):2028–2041. https://doi.org/10.1104/pp.113.222372. (Epub 2013 Jun 25. PMID: 23800991; PMCID: PMC3729780)
Banday ZZ, Cecchini NM, Speed DJ, Scott AT, Parent C, Hu CT, Filzen RC, Agbo E, Greenberg JT (2022) Friend or foe: hybrid proline-rich proteins determine how plants respond to beneficial and pathogenic microbes. Plant Physiol 190(1):860–881. https://doi.org/10.1093/plphys/kiac263. (PMID: 35642916; PMCID: PMC9434206)
Bell E, Mullet JE (1991) Lipoxygenase gene expression is modulated in plants by water deficit, wounding, and methyl jasmonate. Mol Gen Genet 230:456–462
Bell E, Mullet JE (1993) Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol 103:1133–1138
Bienert GP, Schjoerring JK, Jahn TP (2006) Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758:994–1003. https://doi.org/10.1016/j.bbamem.2006.02.015. (Epub 2006 Mar 10 PMID: 16566894)
Blée E (2002) Impact of phytooxylipins in plant defense. Trends Plant Sci 7(7):315–322. https://doi.org/10.1016/s1360-1385(02)02290-2. (PMID: 12119169)
Blée E, Joyard J (1996) Envelope membranes from spinach chloroplasts are a site of metabolism of fatty acid hydroperoxides. Plant Physiol 110:445–454. https://doi.org/10.1104/pp.110.2.445. (PMID: 12226196; PMCID: PMC157739)
Breuers FKH, Bräutigam A, Weber APM (2011) The plastid outer envelope—a highly dynamic interface between plastid and cytoplasm. Front Plant Sci 2:97
Cecchini NM, Steffes K, Schläppi MR, Gifford AN, Greenberg JT (2015) Arabidopsis AZI1 family proteins mediate signal mobilization for systemic defence priming. Nat Commun 6:7658. https://doi.org/10.1038/ncomms8658. (PMID: 26203923)
Cecchini NM, Roychoudhry S, Speed DJ, Steffes K, Tambe A, Zodrow K, Konstantinoff K, Jung HW, Engle NL, Tschaplinski TJ, Greenberg JT (2019) Underground azelaic acid-conferred resistance to Pseudomonas syringae in arabidopsis. Mol Plant Microbe Interact 32(1):86–94. https://doi.org/10.1094/MPMI-07-18-0185-R. (Epub 2018 Oct 23 PMID: 30156481)
Cecchini NM, Speed DJ, Roychoudhry S, Greenberg JT (2021) Kinases and protein motifs required for AZI1 plastid localization and trafficking during plant defense induction. Plant J 105(6):1615–1629. https://doi.org/10.1111/tpj.15137. (Epub 2021 Feb 20. PMID: 33342031; PMCID: PMC8048937)
Conrath U, Beckers GJ, Langenbach CJ, Jaskiewicz MR (2015) Priming for enhanced defense. Annu Rev Phytopathol 53:97–119. https://doi.org/10.1146/annurev-phyto-080614-120132. (Epub 2015 Jun 11 PMID: 26070330)
Davletova S, Schlauch K, Coutu J, Mittler R (2005) The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 139(2):847–856. https://doi.org/10.1104/pp.105.068254. (Epub 2005 Sep 23. PMID: 16183833; PMCID: PMC1256000)
De Domenico S, Bonsegna S, Horres R, Pastor V, Taurino M, Poltronieri P, Imtiaz M, Kahl G, Flors V, Winter P, Santino A (2012) Transcriptomic analysis of oxylipin biosynthesis genes and chemical profiling reveal an early induction of jasmonates in chickpea roots under drought stress. Plant Physiol Biochem 61:115–122. https://doi.org/10.1016/j.plaphy.2012.09.009. (Epub 2012 Oct 11 PMID: 23141673)
Dempsey DA, Klessig DF (2012) SOS - too many signals for systemic acquired resistance? Trends Plant Sci 17(9):538–545. https://doi.org/10.1016/j.tplants.2012.05.011. (Epub 2012 Jun 29 PMID: 22749315)
Dinant S, Lemoine R (2010) The phloem pathway: new issues and old debates. CR Biol 333:307–319
Djami-Tchatchou AT, Ncube EN, Steenkamp PA, Dubery IA (2017) Similar, but different: structurally related azelaic acid and hexanoic acid trigger differential metabolomic and transcriptomic responses in tobacco cells. BMC Plant Biol 17(1):227. https://doi.org/10.1186/s12870-017-1157-5. (PMID: 29187153; PMCID: PMC5706331)
Du H, Liu H, Xiong L (2013) Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci 4:397. https://doi.org/10.3389/fpls.2013.00397. (PMID: 24130566; PMCID: PMC3793129)
Dutton C, Hõrak H, Hepworth C, Mitchell A, Ton J, Hunt L, Gray JE (2019) Bacterial infection systemically suppresses stomatal density. Plant Cell Environ 42(8):2411–2421. https://doi.org/10.1111/pce.13570. (Epub 2019 Jun 10. PMID: 31042812; PMCID: PMC6771828)
Dvorakova L, Cvrckova F, Fischer L (2007) Analysis of the hybrid proline-rich protein families from seven plant species suggests rapid diversification of their sequences and expression patterns. BMC Genom 8:412
El-Shetehy M, Wang C, Shine MB, Yu K, Kachroo A, Kachroo P (2015) Nitric oxide and reactive oxygen species are required for systemic acquired resistance in plants. Plant Signal Behav 10(9):e998544. https://doi.org/10.1080/15592324.2014.998544
Farmer EE, Mueller MJ (2013) ROS-mediated lipid peroxidation and RES-activated signaling. Annu Rev Plant Biol 64:429–450. https://doi.org/10.1146/annurev-arplant-050312-120132. (Epub 2013 Feb 28 PMID: 23451784)
Fernandez-Calvino L, Faulkner C, Walshaw J, Saalbach G, Bayer E, Benitez-Alfonso Y, Maule A (2011) Arabidopsis plasmodesmal proteome. PLoS ONE 6:85. https://doi.org/10.1371/journal.pone.0018880
Froehlich JE, Benning C, Dörmann P (2001a) The digalactosyldiacylglycerol (DGDG) synthase DGD1 is inserted into the outer envelope membrane of chloroplasts in a manner independent of the general import pathway and does not depend on direct interaction with monogalactosyldiacylglycerol synthase for DGDG biosynthesis. J Biol Chem 276:31806–31812. https://doi.org/10.1074/jbc.M104652200
Froehlich JE, Itoh A, Howe GA (2001b) Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipin metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol 125:306–317. https://doi.org/10.1104/pp.125.1.306
Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64:839–863
Fujii J, Soma Y, Matsuda Y (2023) Biological action of singlet molecular oxygen from the standpoint of cell signaling. Injury Death Mol 28(10):4085. https://doi.org/10.3390/molecules28104085. (PMID: 37241826; PMCID: PMC10223444)
Gao QM, Yu K, Xia Y, Shine MB, Wang C, Navarre D, Kachroo A, Kachroo P (2014) Mono- and digalactosyldiacylglycerol lipids function nonredundantly to regulate systemic acquired resistance in plants. Cell Rep 9(5):1681–1691. https://doi.org/10.1016/j.celrep.2014.10.069. (Epub 2014 Nov 26 PMID: 25466253)
Gao QM, Zhu S, Kachroo P, Kachroo A (2015) Signal regulators of systemic acquired resistance. Front Plant Sci 6:228. https://doi.org/10.3389/fpls.2015.00228. (PMID: 25918514; PMCID: PMC4394658)
Gao H, Guo M, Song J, Ma Y, Xu Z (2021) Signals in systemic acquired resistance of plants against microbial pathogens. Mol Biol Rep 48(4):3747–3759. https://doi.org/10.1007/s11033-021-06344-7. (Epub 2021 Apr 24 PMID: 33893927)
Gong Q, Wang Y, He L, Huang F, Zhang D, Wang Y, Wei X, Han M, Deng H, Luo L, Cui F, Hong Y, Liu Y (2023) Molecular basis of methyl-salicylate-mediated plant airborne defence. Nature 622:139–148. https://doi.org/10.1038/s41586-023-06533-3. (Epub 2023 Sep 13 PMID: 37704724)
Gusain S, Joshi S, Joshi R (2023) Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants. Plant Physiol Biochem 197:107646. https://doi.org/10.1016/j.plaphy.2023.107646. (Epub 2023 Mar 15 PMID: 36958153)
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Hartmann M, Kim D, Bernsdorff F, Ajami-Rashidi Z, Scholten N, Schreiber S, Zeier T, Schuck S, Reichel-Deland V, Zeier J (2017) Biochemical principles and functional aspects of pipecolic acid biosynthesis in plant immunity. Plant Physiol 174:124–153
Hartmann M, Zeier T, Bernsdorff F, Reichel-Deland V, Kim D, Hohmann M, Scholten N, Schuck S, Bräutigam A, Hölzel T, Ganter C, Zeier J (2018) Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173(2):456-469.e16. https://doi.org/10.1016/j.cell.2018.02.049. (Epub 2018 Mar 22 PMID: 29576453)
Helle SC, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B (2013) Organization and function of membrane contact sites. Biochim Biophys Acta 1833(11):2526–2541. https://doi.org/10.1016/j.bbamcr.2013.01.028. (Epub 2013 Feb 1 PMID: 23380708)
Howe GA, Schilmiller AL (2002) Oxylipin metabolism in response to stress. Curr Opin Plant Biol 5:230–236. https://doi.org/10.1016/S1369-5266(02)00250-9
Huang Y, Liu Q, Jibrin M, Mou Z, Dufault N, Li Y, Zhang S (2023) Evaluating nicotinamide adenine dinucleotide for its effects on halo blight of snap bean. Plant Dis 107(3):675–681. https://doi.org/10.1094/PDIS-05-22-1126-RE. (Epub 2023 Mar 19 PMID: 35881875)
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47(1):141–153. https://doi.org/10.1093/pcp/pci230. (Epub 2005 Nov 12 PMID: 16284406)
Javvadi SG, Cescutti P, Rizzo R, Lonzarich V, Navarini L, Licastro D, Guarnaccia C, Venturi V (2018) The spent culture supernatant of Pseudomonas syringae contains azelaic acid. BMC Microbiol 28 18(1):199. https://doi.org/10.1186/s12866-018-1352-z
Jose-Estanyol M, Gomis-Ruth FX, Puigdomenech P (2004) The eight-cysteine motif, a versatile structure in plant proteins. Plant Physiol Biochem 42:355–365
Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT (2009) Priming in systemic plant immunity. Science 324(5923):89–91. https://doi.org/10.1126/science.1170025. (PMID: 19342588)
Kachroo A, Kachroo P (2020) Mobile signals in systemic acquired resistance. Curr Opin Plant Biol 58:41–47. https://doi.org/10.1016/j.pbi.2020.10.004. (Epub 2020 Nov 14 PMID: 33202317)
Kim C, Apel K (2013) Singlet oxygen-mediated signaling in plants: moving from flu to wild type reveals an increasing complexity. Photosynth Res 116:455–464
Kim TJ, Lim GH (2023) Salicylic acid and mobile regulators of systemic immunity in plants: transport and metabolism. Plants 12(5):1013. https://doi.org/10.3390/plants12051013. (PMID: 36903874; PMCID: PMC10005269)
Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2008) Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry 69:2127–2132. https://doi.org/10.1016/j.phytochem.2008.04.023
Koch E, Meier BM, Eiben HG, Slusarenko A (1992) A lipoxygenase from leaves of tomato (Lycopersicon esculentum Mill.) is induced in response to plant pathogenic pseudomonads. Plant Physiol 99(2):571–576. https://doi.org/10.1104/pp.99.2.571
Korenblum E, Dong Y, Szymanski J, Panda S, Jozwiak A, Massalha H, Meir S, Rogachev I, Aharoni A (2020) Rhizosphere microbiome mediates systemic root metabolite exudation by rootto-root signaling. Proc Natl Acad Sci U S A 117(7):3874–3883. https://doi.org/10.1073/pnas.1912130117
Kundu A, Mishra S, Kundu P, Jogawat A, Vadassery J (2022) Piriformospora indica recruits host-derived putrescine for growth promotion in plants. Plant Physiol 28 188(4):2289–2307. https://doi.org/10.1093/plphys/kiab536
Lee JY, Wang X, Cui W, Sager R, Modla S, Czymmek K, Zybaliov B, van Wijk K, Zhang C, Lu H, Lakshmanan V (2011) A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 23(9):3353–3373. https://doi.org/10.1105/tpc.111.087742. (Epub 2011 Sep 20. PMID: 21934146; PMCID: PMC3203451)
Li M, Kim C (2021) Chloroplast ROS and stress signaling. Plant Commun 3(1):100264. https://doi.org/10.1016/j.xplc.2021.100264. (PMID: 35059631; PMCID: PMC8760138)
Li CH, Wang G, Zhao JL, Zhang LQ, Ai LF, Han YF, Sun DY, Zhang SW, Sun Y (2014) The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice. Plant Cell 26(6):2538–2553. https://doi.org/10.1105/tpc.114.125187. (Epub 2014 Jun 6. PMID: 24907341; PMCID: PMC4114950)
Li T, Xiao Z, Li H, Liu C, Shen W, Gao C (2020) A combinatorial reporter set to visualize the membrane contact sites between endoplasmic reticulum and other organelles in plant cell. Front Plant Sci 11:1280
Lim GH, Shine MB, de Lorenzo L, Yu K, Cui W, Navarre D, Hunt AG, Lee JY, Kachroo A, Kachroo P (2016) Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host Microbe 19(4):541–549. https://doi.org/10.1016/j.chom.2016.03.006. (PMID: 27078071)
Liu W, Wang H, Chen Y, Zhu S, Chen M, Lan X, Chen G, Liao Z (2017) Cold stress improves the production of artemisinin depending on the increase in endogenous jasmonate. Biotechnol Appl Biochem 64(3):305–314. https://doi.org/10.1002/bab.1493. (Epub 2016 Oct 10 PMID: 26988377)
Losvik A, Beste L, Glinwood R, Ivarson E, Stephens J, Zhu LH, Jonsson L (2017) Overexpression and down-regulation of barley lipoxygenase LOX2.2 affects jasmonate-regulated genes and aphid fecundity. Int J Mol Sci 18(12):2765. https://doi.org/10.3390/ijms18122765
Maruyama K, Sakuma Y, Kasuga M, Ito Y, Seki M, Goda H, Shimada Y, Yoshida S, Shinozaki K, Yamaguchi-Shinozaki K (2004) Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J 38(6):982–993. https://doi.org/10.1111/j.1365-313X.2004.02100.x. (PMID: 15165189)
Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9:274–280
Miranda de la Torre JO, Peppino Margutti MY, Lescano López I, Cambiagno DA, Alvarez ME, Cecchini NM (2023) The Arabidopsis chromatin regulator MOM1 is a negative component of the defense priming induced by AZA. BABA and PIP Front Plant Sci 14:1133327. https://doi.org/10.3389/fpls.2023.1133327. (PMID:37229135;PMCID:PMC10203520)
Mitra SK, Walters BT, Clouse SD, Goshe MB (2009) An efficient organic solvent based extraction method for the proteomic analysis of arabidopsis plasma membranes. J Proteom Res 8:2752–2767. https://doi.org/10.1021/pr801044y
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mosblech A, Feussner I, Heilmann I (2009) Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem 47:511–517
Nieto-Garibay A, Barraza A, Caamal-Chan G, Murillo-Amador B, Troyo-Diéguez E, Burgoa-Cruz CA, Jaramillo-Limón JN, Loera-Muro A (2022) Habanero pepper (Capsicum chinense) adaptation to water-deficit stress in a protected agricultural system. Funct Plant Biol 49(3):295–306. https://doi.org/10.1071/FP20394. (PMID: 35130477)
Oelmüller R (2021) Threat at one end of the plant: What travels to inform the other parts? Int J Mol Sci 22(6):3152. https://doi.org/10.3390/ijms22063152.PMID:33808792;PMCID:PMC8003533
Ohta H, Shida K, Peng YL, Furusawa I, Shishiyama J, Aibara S, Morita Y (1991) A lipoxygenase pathway is activated in rice after infection with the rice blast fungus Magnaporthe grisea. Plant Physiol 97(1):94–98. https://doi.org/10.1104/pp.97.1.94.PMID:16668421;PMCID:PMC1080968
Panda A, Rangani J, Parida AK (2021) Unraveling salt responsive metabolites and metabolic pathways using non-targeted metabolomics approach and elucidation of salt tolerance mechanisms in the xero-halophyte Haloxylon salicornicum. Plant Physiol Biochem 158:284–296. https://doi.org/10.1016/j.plaphy.2020.11.012. (Epub 2020 Nov 16 PMID: 33239222)
Pitzschke A, Schikora A, Hirt H (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12(4):421–426. https://doi.org/10.1016/j.pbi.2009.06.008. (Epub 2009 Jul 14 PMID: 19608449)
Pitzschke A, Datta S, Persak H (2014a) Mitogen-activated protein kinase-regulated AZI1 - an attractive candidate for genetic engineering. Plant Signal Behav 9(2):e27764. https://doi.org/10.4161/psb.27764. (Epub 2014 Feb 10. PMID: 24518841; PMCID: PMC4091252)
Pitzschke A, Datta S, Persak H (2014b) Salt stress in Arabidopsis: lipid transfer protein AZI1 and its control by mitogen-activated protein kinase MPK3. Mol Plant 7(4):722–738. https://doi.org/10.1093/mp/sst157. (Epub 2013 Nov 8. PMID: 24214892; PMCID: PMC3973493)
Pitzschke A, Xue H, Persak H, Datta S, Seifert GJ (2016) Post-translational modification and secretion of azelaic acid induced 1 (AZI1), a hybrid proline-rich protein from arabidopsis. Int J Mol Sci 17(1):85. https://doi.org/10.3390/ijms17010085. (PMID: 26771603; PMCID: PMC4730328)
Pospíšil P (2016) Production of reactive oxygen species by photosystem ii as a response to light and temperature stress. Front Plant Sci 7:1950. https://doi.org/10.3389/fpls.2016.01950. (PMID: 28082998; PMCID: PMC5183610)
Prost I, Dhondt S, Rothe G, Vicente J, Rodriguez MJ, Kift N, Carbonne F, Griffiths G, Esquerré-Tugayé MT,
Radi R (1998) Peroxynitrite reactions and diffusion in biology. Chem Res Toxicol 11:720–721
Riedlmeier M, Ghirardo A, Wenig M, Knappe C, Koch K, Georgii E, Dey S, Parker JE, Schnitzler JP, Vlot AC (2017) Monoterpenes Support Systemic Acquired Resistance within and between Plants. Plant Cell 29(6):1440–1459. https://doi.org/10.1105/tpc.16.00898. (Epub 2017 May 23. PMID: 28536145; PMCID: PMC5502447)
Rodriguez MC, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649. https://doi.org/10.1146/annurev-arplant-042809-112252. (PMID: 20441529)
Rosahl S, Castresana C, Hamberg M, Fournier J (2005) Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol 139(4):1902–1913. https://doi.org/10.1104/pp.105.066274. (Epub 2005 Nov 18. PMID: 16299186; PMCID: PMC1310568)
Rustgi S, Springer A, Kang C, von Wettstein D, Reinbothe C, Reinbothe S, Pollmann S (2019) Allene oxide synthase and hydroperoxide lyase, two non-canonical cytochrome P450s in Arabidopsis thaliana and their different roles in plant defense. Int J Mol Sci 20(12):3064. https://doi.org/10.3390/ijms20123064. (PMID: 31234561; PMCID: PMC6627107)
Ryter SW, Tyrrell RM (1998) Singlet molecular oxygen (1O2): a possible effector of eukaryotic gene expression. Free Radic Biol Med 24(9):1520–1534. https://doi.org/10.1016/s0891-5849(97)00461-9. (PMID: 9641271)
Saikia B, Singh S, Debbarma J, Velmurugan N, Dekaboruah H, Arunkumar KP, Chikkaputtaiah C (2020) Multigene CRISPR/Cas9 genome editing of hybrid proline rich proteins (HyPRPs) for sustainable multi-stress tolerance in crops: the review of a promising approach. Physiol Mol Biol Plants 26(5):857–869. https://doi.org/10.1007/s12298-020-00782-6. (Epub 2020 Apr 20. PMID: 32377037; PMCID: PMC7196567)
Shi LN, Lu LX, Ye JR, Shi HM (2022) The endophytic strain ZS-3 enhances salt tolerance in Arabidopsis thaliana by regulating photosynthesis, osmotic stress, and ion homeostasis and inducing systemic tolerance. Front Plant Sci 13:820837. https://doi.org/10.3389/fpls.2022.820837. (PMID: 35386673; PMCID: PMC8977589)
Shine MB, Xiao X, Kachroo P, Kachroo A (2019) Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci 279:81–86. https://doi.org/10.1016/j.plantsci.2018.01.001. (Epub 2018 Jan 3 PMID: 30709496)
Siebers M, Brands M, Wewer V, Duan Y, Hölzl G, Dörmann P (2016) Lipids in plant-microbe interactions. Biochim Biophys Acta 1861:1379–1395. https://doi.org/10.1016/j.bbalip.2016.02.021
Siedow JN (1991) Plant lipoxygenase: structure and function. Annu Rev Plant Physiol Plant Mol Biol 42:145–188
Takata T, Araki S, Tsuchiya Y, Watanabe Y (2020) Oxidative stress orchestrates mapk and nitric-oxide synthase signal. Int J Mol Sci 21(22):8750. https://doi.org/10.3390/ijms21228750. (PMID: 33228180; PMCID: PMC7699490)
Toulmay A, Prinz WA (2011) Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol 23:458–463
Triantaphylides C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148:960–968
Triantaphylidès C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14(4):219–228. https://doi.org/10.1016/j.tplants.2009.01.008. (Epub 2009 Mar 18 PMID: 19303348)
Tripathy BC, Oelmüller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7(12):1621–1633. https://doi.org/10.4161/psb.22455. (Epub 2012 Oct 16. PMID: 23072988; PMCID: PMC3578903)
Vandelle E, Delledonne M (2011) Peroxynitrite formation and function in plants. Plant Sci 81:534–539
Vellosillo T, Martínez M, López MA, Vicente J, Cascón T, Dolan L, Hamberg M, Castresana C (2007) Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 19(3):831–846. https://doi.org/10.1105/tpc.106.046052. (Epub 2007 Mar 16. PMID: 17369372; PMCID: PMC1867370)
Vick BA, Zimmerman DC (1987) The Biochemistry of Plants, ed. Stumpf, P. K. (Academic, New York), Vol. 9:53–90
Vlot AC, Sales JH, Lenk M, Bauer K, Brambilla A, Sommer A, Chen Y, Wenig M, Nayem S (2021) Systemic propagation of immunity in plants. New Phytol 229(3):1234–1250. https://doi.org/10.1111/nph.16953. (Epub 2020 Oct 24 PMID: 32978988)
Wang Z, Benning C (2012) Chloroplast lipid synthesis and lipid trafficking through ER-plastid membrane contact sites. Biochem Soc Trans 40:457–463
Wang X, Sager R, Cui W, Zhang C, Lu H, Lee JY (2013) Salicylic acid regulates Plasmodesmata closure during innate immune responses in Arabidopsis. Plant Cell 25(6):2315–2329. https://doi.org/10.1105/tpc.113.110676. (Epub 2013 Jun 7. PMID: 23749844; PMCID: PMC3723628)
Wang C, El-Shetehy M, Shine MB, Yu K, Navarre D, Wendehenne D, Kachroo A, Kachroo P (2014) Free radicals mediate systemic acquired resistance. Cell Rep 7(2):348–355. https://doi.org/10.1016/j.celrep.2014.03.032. (Epub 2014 Apr 13 PMID: 24726369)
Wang XY, Li DZ, Li Q, Ma YQ, Yao JW, Huang X, Xu ZQ (2016) Metabolomic analysis reveals the relationship between AZI1 and sugar signaling in systemic acquired resistance of Arabidopsis. Plant Physiol Biochem 107:273–287. https://doi.org/10.1016/j.plaphy.2016.06.016. (Epub 2016 Jun 15 PMID: 27337039)
Wang C, Liu R, Lim GH, de Lorenzo L, Yu K, Zhang K, Hunt AG, Kachroo A, Kachroo P (2018) Pipecolic acid confers systemic immunity by regulating free radicals. Sci Adv 4(5):eaar4509. https://doi.org/10.1126/sciadv.aar4509. (PMID: 29854946; PMCID: PMC5976275)
Wendehenne D, Gao QM, Kachroo A, Kachroo P (2014) Free radical-mediated systemic immunity in plants. Curr Opin Plant Biol 20:127–134. https://doi.org/10.1016/j.pbi.2014.05.012. (Epub 2014 Jun 12 PMID: 24929297)
Wittek F, Hoffmann T, Kanawati B, Bichlmeier M, Knappe C, Wenig M, Schmitt-Kopplin P, Parker JE, Schwab W, Vlot AC (2014) Arabidopsis ENHANCED DISEASE SUSCEPTIBILITY1 promotes systemic acquired resistance via azelaic acid and its precursor 9-oxo nonanoic acid. J Exp Bot 65(20):5919–5931. https://doi.org/10.1093/jxb/eru331. (Epub 2014 Aug 11. PMID: 25114016; PMCID: PMC4203127)
Wong CE, Li Y, Labbe A, Guevara D, Nuin P, Whitty B, Diaz C, Golding GB, Gray GR, Weretilnyk EA, Griffith M, Moffatt BA (2006) Transcriptional profiling implicates novel interactions between abiotic stress and hormonal responses in Thellungiella, a close relative of Arabidopsis. Plant Physiol 140(4):1437–1450. https://doi.org/10.1104/pp.105.070508. (Epub 2006 Feb 24. PMID: 16500996; PMCID: PMC1435811)
Xu ZY, Zhang X, Schläppi M, Xu ZQ (2011) Cold-inducible expression of AZI1 and its function in improvement of freezing tolerance of Arabidopsis thaliana and Saccharomyces cerevisiae. J Plant Physiol 168(13):1576–1587. https://doi.org/10.1016/j.jplph.2011.01.023. (Epub 2011 Apr 14 PMID: 21492954)
Yu K, Soares JM, Mandal MK, Wang C, Chanda B, Gifford AN, Fowler JS, Navarre D, Kachroo A, Kachroo P (2013) A feedback regulatory loop between G3P and lipid transfer proteins DIR1 and AZI1 mediates azelaic-acid-induced systemic immunity. Cell Rep 3(4):1266–1278. https://doi.org/10.1016/j.celrep.2013.03.030. (Epub 2013 Apr 18 PMID: 23602565)
Zhang Y, Schläppi M (2007) Cold responsive EARLI1 type HyPRPs improve freezing survival of yeast cells and form higher order complexes in plants. Planta 227(1):233–243. https://doi.org/10.1007/s00425-007-0611-2. (Epub 2007 Sep 5 PMID: 17786468)
Zhao Q, Liu F, Song C, Zhai T, He Z, Ma L, Zhao X, Jia Z, Song S (2023) Diffusible signal factor primes plant immunity against Xanthomonas campestris pv. campestris (Xcc) via JA signaling in Arabidopsis and Brassica oleracea. Front Cell Infect Microbiol 13:1203582. https://doi.org/10.3389/fcimb.2023.1203582. (PMID: 37404719; PMCID: PMC10315614)
Zoeller M, Stingl N, Krischke M, Fekete A, Waller F, Berger S, Mueller MJ (2012) Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: biogenesis of pimelic and azelaic acid. Plant Physiol 160(1):365–378. https://doi.org/10.1104/pp.112.202846. (Epub 2012 Jul 22. PMID: 22822212; PMCID: PMC3440211)
Acknowledgements
None
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding was received from the Deutsche Forschungsgemeinschaft (CRC1127, project ID: 239748522 to R.O.) PR was supported by the Indian Council of Agricultural Research, New Delhi, India, and the International Max-Planck Research School (Chemical Ecology, Jena).
Author information
Authors and Affiliations
Contributions
Conceptualization: PR. Writing of manuscript: PR and RO.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Priya Reddy, Y.N., Oelmüller, R. Lipid peroxidation and stress-induced signalling molecules in systemic resistance mediated by azelaic acid/AZELAIC ACID INDUCED1: signal initiation and propagation. Physiol Mol Biol Plants 30, 305–316 (2024). https://doi.org/10.1007/s12298-024-01420-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-024-01420-1