Abstract
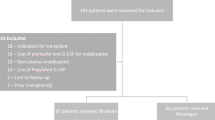
No consensus has been made on the use of PEG-modification recombinant human granulocyte colony stimulating factor (PEG-rhG-CSF) in patients receiving autologous peripheral blood stem cell transplantation (PBSCT). To evaluate the efficacy and safety of PEG-rhG-CSF in provision of neutrophil support for lymphoma patients receiving autologous PBSCT. This retrospective study included lymphoma patients receiving either PEG-rhG-CSF or rhG-CSF after autologous PBSCT from 2018 to 2021 in two clinics. Hematologic recovery time, incidence of infectious complications and toxicity were compared between these two rhG-CSFs and among different initiation time of PEG-rhG-CSF. Of the 139 subjects included, 93 received PEG-rhG-CSF and 46 received rhG-CSF after transplantation. Compared with rhG-CSF, PEG-rhG-CSF marginally but significantly accelerated the neutrophil engraftment by 1 day (10 vs. 9 days, respectively) with no increasing on the risk of infectious complication and toxicity. In the PEG-rhG-CSF group, 50 patients received the growth factor on day 1, 19 received on day 3 and 24 received on day 5. The neutrophil engraftment was significantly shorter in day 1 and day 3 subgroup (9, 9, and 10 days, respectively), with a lower incidence of febrile neutropenia (82%, 100%, 100%) and documented infections (76%, 100%, 100%) in day 1 subgroup. PEG-rhG-CSF might be an alternative to rhG-CSF for lymphoma patients received autologous PBSCT. Administrating PEG-rhG-CSF on day 1 can achieve both faster hematologic recovery and lower infectious complications.


Similar content being viewed by others
References
Aapro M, Boccia R, Leonard R et al (2017) Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer 25:3295–3304
Holmes FA, O’Shaughnessy JA, Vukelja S et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20:727–731
Xie J, Cao J, Wang JF et al (2018) Advantages with prophylactic PEG-rhG-CSF versus rhG-CSF in breast cancer patients receiving multiple cycles of myelosuppressive chemotherapy: an open-label, randomized, multicenter phase III study. Breast Cancer Res Treat 168:389–399
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol 33:3199–3212
Maiolino A, Biasoli I, Lima J, Portugal AC, Pulcheri W, Nucci M (2003) Engraftment syndrome following autologous hematopoietic stem cell transplantation: definition of diagnostic criteria. Bone Marrow Transpl 31:393–397
Klumpp TR, Mangan KF, Goldberg SL, Pearlman ES, Macdonald JS (1995) Granulocyte colony-stimulating factor accelerates neutrophil engraftment following peripheral-blood stem-cell transplantation: a prospective, randomized trial. J Clin Oncol 13:1323–1327
Schmitz N, Ljungman P, Cordonnier C et al (2004) Lenograstim after autologous peripheral blood progenitor cell transplantation: results of a double-blind, randomized trial. Bone Marrow Transpl 34:955–962
Singh AD, Parmar S, Patel K et al (2018) Granulocyte colony-stimulating factor use after autologous peripheral blood stem cell transplantation: comparison of two practices. Biol Blood Marrow Transpl 24:288–293
Ziakas PD, Kourbeti IS (2012) Pegfilgrastim vs. filgrastim for supportive care after autologous stem cell transplantation: can we decide? Clin Transpl 26(1):16–22
Sebban C, Lefranc A, Perrier L et al (2012) A randomised phase II study of the efficacy, safety and cost-effectiveness of pegfilgrastim and filgrastim after autologous stem cell transplant for lymphoma and myeloma (PALM study). Eur J Cancer 48:713–720
Ferrara F, Izzo T, Criscuolo C et al (2011) Comparison of fixed dose pegfilgrastim and daily filgrastim after autologous stem cell transplantation in patients with multiple myeloma autografted on a outpatient basis. Hematol Oncol 29:139–143
Sheth V, Gore A, Jain R, Ghanekar A, Saikia T (2019) Pegfilgrastim: more cost effective and equally efficacious option as compared to filgrastim in autologous stem cell transplant. Indian J Hematol Blood Transfus 35:66–71
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors stated that they had no interests which might be perceived as posing a conflict or bias.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Li, J., Yang, P. et al. The Effectiveness and Optimal Timing of PEG-rhG-CSF After Autologous Peripheral Blood Stem Cell Transplantation: A Multicenter Experience. Indian J Hematol Blood Transfus (2023). https://doi.org/10.1007/s12288-023-01704-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12288-023-01704-8