Abstract
Purpose of Review
In this narrative review, we discuss recent literature regarding early antifungal therapy in critically ill patients, focusing in particular on the current role of empirical antifungal treatment.
Recent Findings
While the direction of effect in randomized controlled trials (RCTs) exploring efficacy of empirical therapy in intensive care unit (ICU) patients with suspected invasive candidiasis (IC) was most frequently toward a favorable impact of empirical therapy, no formal demonstration of superiority was observed.
Summary
Main results from RCTs seem in contrast with the increased mortality reported from observational studies in case of delayed antifungal therapy in patients with IC, suggesting, in our opinion, that further research is still necessary to better delineate the precise subgroup of ICU patients with suspected IC who may benefit from early antifungal therapy, either early empirical based on risk scores or diagnostic-driven, or a combination of both.
Similar content being viewed by others
Introduction
Invasive candidiasis (IC) is a spectrum of diseases including candidemia and deep-seated candidiasis [1, 2•]. Critically ill patients are among those at the highest risk of developing IC, and approximately one-third of all candidemia episodes are diagnosed in intensive care units (ICUs) [3, 4].
Candidemia is the most common form of IC, with a reported prevalence of 6.9 episodes per 1000 ICU patients [5]. With regard to deep-seated candidiasis, intra-abdominal candidiasis (IAC) accounts for most cases [6]. Other forms of deep-seated candidiasis observed more rarely in ICU patients include hematogenous disseminated disease such as hepatosplenic, ocular, cardiac, central nervous system, bone and renal candidiasis, which are seen more frequently in patients with prolonged candidemia, and/or in immunosuppressed patients [7].
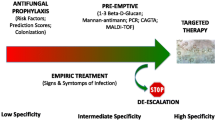
Early antifungal treatment (before etiological diagnosis by classical culture-based methods) could be crucial in critically ill patients with suspected IC, since delays in treatment have been associated with increased mortality in those who truly have IC [8,9,10,11,12]. On the other hand, overtreatment may be harmful, exposing patients with a clinical presentation compatible with IC, but actually caused by other organisms (usually bacteria), to unnecessary drug toxicities and increased risk of emergence of antifungal resistance in commensal Candida isolates. In the attempt to find the right balance between overtreatment and undertreatment of critically ill patients with suspected IC, various early antifungal strategies have been proposed: (i) risk score-based empirical antifungal therapy; (ii) diagnostic-driven antifungal therapy based on the results of rapid non-culture-based tests; and (iii) pre-emptive therapy based on results of non-culture based tests performed as screening measure in patients at risk of IC but without manifest signs and symptoms of infection.
In this narrative review, we discuss recent literature regarding early antifungal therapy in critically ill patients, focusing in particular on the current role of empirical antifungal treatment.
Methods
A PubMed search was conducted for English language articles published before 1 December 2023, using various combinations of the key words “invasive candidiasis”, “critically ill” and “empirical treatment”, with particular focus on the last 5 years. Articles pertinent to the topic were selected for full text review based on authors’ judgment, and references of selected articles were also screened for retrieving further pertinent literature, focusing in particular on results from randomized controlled trials (RCTs). Eventually, the present narrative review was structured in the following sections: (i) a brief background regarding epidemiology, prognostic impact, and diagnosis of IC in the ICU; (ii) a discussion of current literature regarding early treatment of IC in the ICU; (iii) conclusions (including discussion of previous sections).
Background
Epidemiology and Prognostic Impact of Invasive Candidiasis in the ICU
IC remains frequent and associated with poor outcomes in the ICU, as reported in a large multinational, multicenter, retrospective study conducted in 23 ICUs across different European countries (EUCANDICU project), in which the cumulative incidence of IC was 7.07 episodes per 1000 ICU admissions with a crude 30-day mortality of 42% [13]. The non-mutually exclusive cumulative incidences of candidemia and IAC were 5.52 and 1.84 episodes per 1000 ICU admissions, respectively [13]. In the observational, prospective, multicenter EUROBACT study, conducted in 162 ICUs in 24 countries; 28-day mortality of candidemia was as high as 41% [14].
The epidemiology of IC has evolved in recent years, with a progressive shift from C. albicans to non-albicans Candida spp. observed globally, although it should be acknowledged that the shift was less certain or marked when the analysis was limited to ICU wards in a recent meta-analysis and meta-regression [15,16,17,18]. In the EUROBACT study, the relative frequency of C. albicans as causative agent of candidemia was 57%, followed by C. glabrata and C. parapsilosis, while in the more recent EUROBACT-2 study, C. albicans was responsible for 40% of fungemia episodes, and 58% were caused by non-albicans Candida species [14, 19•]. In the prospective, observational EPIC III study, involving 8135 ICU patients with suspected or proven infection from 1150 centers in 88 countries, C. albicans was isolated from 10% of patients with positive microbiological samples, while non-albicans species were 5% of the isolates [20].
Regarding antifungal resistance, in the SENTRY Antifungal Surveillance Program, including 20,788 Candida spp. isolates from different continents, fluconazole resistance in the most frequent Candida species ranged from 0.3% (C. albicans) to 8.1% (C. glabrata) [15]. Resistance to echinocandins was uncommon among isolates of C. albicans (0.0–0.1%), C. parapsilosis (0.0–0.1%), C. tropicalis (0.5–0.7%), and C. krusei (0.0–1.7%) [15]. Echinocandin-resistant C. glabrata has been more frequently reported from the USA (reported prevalence up to 12% in some centers) [21, 22], with cross-resistance to fluconazole in up to one-third of isolates [23], than from Europe [15]. In addition, the emergent pathogen C. auris has caused outbreaks in ICU wards worldwide, posing a complex challenge with regard to IC management [24•, 25]. Indeed, C. auris isolates are usually resistant to fluconazole and they show variable amphotericin and echinocandin susceptibility, with concomitant resistance to azoles, echinocandins, and polyenes having been reported [26,27,28].
Brief Diagnostic Considerations
Microscopic examination is rapid and can be helpful but a negative result does not exclude IC [29]. Cultures are the diagnostic gold standard but are positive in only 50–70% of cases of candidemia, and even less frequently in IAC (5–20%) [30, 31]. Furthermore, it can take several days before Candida is identified at species level and antifungal susceptibility test results are available. Recently, non-culture-based blood tests have been developed for detection of components of the fungal cell wall by immunoassays, DNA by PCR, and antibodies by serology. Their role in guiding diagnostic-driven early antifungal therapy is discussed below.
Early Antifungal Therapy of IC in the ICU
Risk Score-Based Empirical Antifungal Therapy
Numerous risk factors for IC have been identified, for example high Acute Physiology and Chronic Health Evaluation II (APACHE II) score, diabetes mellitus, renal insufficiency, surgery (especially abdominal surgery), pancreatitis, the use of broad-spectrum antibiotics, parenteral nutrition, hemodialysis, mechanical ventilation, presence of central vascular catheters, previous Candida colonization, and therapy with immunosuppressive agents [3, 32,33,34,35]. These risk factors have been condensed into various prediction scores (or risk scores) validated in clinical studies [36,37,38,39,40,41,42,43]. Risk scores can be considered as early diagnostic tools when evaluating empirical antifungal therapy in ICU at the bedside of patients with signs and symptoms consistent with IC. However, one challenge is that most risk factors for IC are also common among ICU patients without IC. Accordingly, risk scores usually demonstrate high negative predictive value (NPV), while their positive predictive value (PPV) is usually low [44].
Risk scores based on the burden of Candida colonization were initially proposed in the twentieth century to guide empirical antifungal therapy [38, 45, 46], while many other scores based only on clinical variables or on a combination of clinical variables and Candida colonization have been proposed subsequently. Some well-known risk scores are available in the literature. For example, Ostrosky‐Zeichner and colleagues developed a clinical prediction rule for IC associated with a 10% risk of IC, and 97% NPV [40]. Such a risk is defined in the presence of the following items: any systemic antibiotic OR presence of a central venous catheter AND at least two of the following: total parenteral nutrition, any dialysis, any major surgery, pancreatitis, any use of steroids or use of other immunosuppressive agents [40]. Paphitou and colleagues also developed a prediction rule conferring a 20% risk of IC to any ICU patient with at least one among diabetes mellitus, new onset hemodialysis, and use of total parenteral nutrition, plus ICU stay longer than 4 days, use of broad-spectrum antibiotics, and no receipt of antifungals [39]. According to the Candida score, developed by Leon and colleagues, a cumulative score of > 2.5 points was suggested as a possible cutoff for initiating empirical antifungals (based on a risk ratio of 7.35). Items defining the score included the following: multifocal Candida colonization (1 point); total parenteral nutrition (1 point); surgery as the reason of ICU admission (1 point); and clinical symptoms of severe sepsis (2 points) [41]. Guillamet developed a score based on clinical variables for predicting the risk of candidemia in a large retrospective cohort of patients with severe sepsis or septic shock [47]. The authors identified administration of total parenteral nutrition (+ 2 points), prior antibiotic exposure (+ 2 points), transfer from an outside hospital (+ 1 points) or admission from a nursing home (+ 2 points), mechanical ventilation (+ 1 points), and presence of a central venous catheter (+ 2 points) as independent predictors of candidemia, while the lung as the presumed source of sepsis was conferred -6 points [47]. Overall, the risk reached 43% for + 8 points [47]. In a prospective cohort study of nonneutropenic patients admitted to the ICU for ≥ 72 h, Playford and colleagues identified the following factors to be independently associated with an increased risk of IC: emergency gastrointestinal or hepatobiliary surgical procedure; ICU admission from operating theater, emergency department, or another hospital; presence of an uncoated central venous catheter; total parenteral nutrition; high-dose corticosteroids; prior antibiotic exposure; and prior Candida colonization of throat or urine [48]. The authors identified three patient cohorts based on two threshold scores: patients at high risk (PPV 11.7%); patients at intermediate risk (PPV 1.46%); and patients at low risk (PPV 0.24%) [48].
Regarding current evidence from RCTs on the use of an empirical treatment approach to IC in critically ill patients, EMPIRICUS was a multicenter double-blind randomized controlled trial (RCT) conducted in 260 nonneutropenic critically ill patients with ICU-acquired sepsis, multiple Candida colonization, multiple organ failure, and exposed to broad-spectrum antibacterial agents that compared empirical micafungin vs. placebo [49]. The primary time-to-event endpoint was invasive fungal infection-free survival at day 28. Of note, this endpoint highlights the frequent dichotomy observed in RCTs in this field regarding the aims of the administered antifungal therapy: (i) to improve survival of patients with signs and symptoms of infection (in this case, ICU-acquired sepsis) as empirical therapy; (ii) to prevent development of future IC events, as prophylactic therapy. Overall, despite a trend toward a favorable effect of empirical antifungal therapy, invasive fungal infection-free survival at day 28 was not significantly different in the micafungin arm vs. placebo arm, both in the entire study population (hazard ratio [HR] 1.35, 95% confidence interval [CI] 0.87–2.08]) and in the subgroup of patients with high (> 8 points) sequential organ failure assessment (SOFA) score (HR 1.69,95% CI 0.96–2.94) [49]. The INTENSE trial was another RCT evaluating early antifungal therapy with micafungin vs. placebo in critically ill patients with intra-abdominal infection requiring emergency surgery [50]. Of note, antifungal therapy was defined as “pre-emptive” in this study since the primary outcome was incidence of IC, although from this standpoint it could be considered as prophylaxis rather than preemptive therapy (i.e., antifungal agents to prevent future IC without considering results of screening non-culture-based tests). Death occurred in 31/122 (25.4%) patients in the micafungin arm and in 28/126 (22.2%) patients in the placebo arm in the safety analysis set (which also included confirmed IC at baseline, registered in 2 and 5 patients in the micafungin and placebo arms, respectively) [50]. In a double-blind RCT conducted in 270 ICU patients with persistent fever despite administration of broad spectrum antibiotics, empirical intravenous fluconazole was compared to placebo with respect to a primary efficacy endpoint of success in all the following: (i) resolution of fever; (ii) absence of invasive fungal infection (iii) no discontinuation owing to toxicity; and (iv) no need for other systemic antifungal medications [51]. Of note, all patients had a high (> 16) APACHE II score and central venous catheters. Overall, achievement of the primary endpoint was registered in 36% (44/122) and 38% (48/127) of patients in fluconazole and placebo arms, respectively (relative risk 0.95, with 95% CI from 0.69 to 1.32) [51].
A summary of results from RCTs on the efficacy of empirical antifungal therapy in ICU patients with suspected IC is also available in Table 1.
Diagnostic-Driven Early Antifungal Therapy
Theoretically, rapid non-culture-based tests offer two potential advantages in terms of early antifungal therapy in ICU patients with suspected IC: (i) they improve overall PPV for IC in patients with risk scores suggesting IC; (ii) they usually show a high NPV on their own, thereby potentially allowing safe non-initiation or rapid discontinuation of antifungals independent of risk scores results. Various non-culture-based tests have been evaluated for their diagnostic performance in ICU patients, for example, serum 1,3-β-D-glucan (BDG), T2 magnetic resonance Candida assay (T2Candida), multiplex Candida real-time PCR, the combination of mannan antigen (Mn) and anti-mannan IgG antibodies (Anti-Mn), and Candida albicans germ tube antibody (CAGTA). An advantage of these diagnostic tools is their quick turnaround time compared to classical cultures, although they could better be defined as Bayesian biomarkers as they confer a probability of infection and not a definitive diagnosis [30]. For this latter reason, non-culture-based tests should be utilized and interpreted based on pre-test probability of IC (in turn, arising from the combination of clinical picture and baseline risk according to risk factors) [52, 53•, 54].
The most studied non-culture-based test for the diagnosis of IC in critically ill patients is serum BDG, a nearly pan fungal antigen test which has shown variable sensitivity and specificity (mostly around 70–80% and 55–60%, respectively) [53•, 55]. Serum BDG displays an excellent NPV, while its specificity and PPV have been reported to improve with consecutive sampling and by increasing the positivity cut-off (though with the risks of delaying diagnosis and reducing sensitivity and NPV, respectively) [56]. Another suggested way to increase the PPV of BDG in ICU patients with suspected candidemia is to combine serum BDG with other either specific or nonspecific markers of IC, such as low procalcitonin, although the related evidence is still limited to a few observational studies and deserves further investigation [57, 58]. An improved cost-effectiveness of guiding early antifungal therapy by serum BDG results vs. empirical therapy of critically ill patients with suspected candidemia and high Candida score (at least 3 points) has been proposed by using desirability of outcome ranking (DOOR) analyses, although also in this case evidence is still limited and observational [59]. Furthermore, results of DOOR analyses could be complex to interpret at the bedside, by overall suggesting the probability of a “better choice” rather than classical probability of diagnosis [60]. If further explored and validated in the future, the results of DOOR analyses for the early diagnosis of IC could be considered in conjunction with those of classical analyses, with the aim of presenting a more comprehensive evaluation of the patients’ baseline risk of IC to clinicians. Finally, two other general considerations should be made regarding serum BDG as an early diagnostic tool for IC: (i) sensitivity of serum BDG seems to vary according to Candida species, with the lowest having been reported for C. parapsilosis and C. auris [61,62,63]; (ii) there is increasing interest in combining the use of serum BDG with peritoneal BDG in critically ill patients with suspected IAC, with a recent, prospective multicenter study suggesting high NPV in case of low concentrations of both serum BDG and peritoneal BDG [64•].
Regarding molecular methods (including also the T2Candida assay), the experience in critically ill patients is still limited, but certainly their diagnostic performance in this specific population deserves further dedicated investigation [53•, 65,66,67]. Notably, while they are correctly classified as “non-culture-based tests”, they also enable etiological diagnosis at species level, in contrast to antigen-based tests. This could represent a potential advantage, although high quality evidence from well designed RCTs is needed before widespread adoption in clinical practice. Regarding current evidence from RCTs on the use of a diagnostic-driven approach to IC in critically ill patients, the CandiSep trial was a multicenter RCT comparing clinical outcomes of a serum BDG-driven approach versus a culture-driven approach to antifungal prescribing in ICU patients with sepsis or septic shock and at risk of IC (risk defined as total parenteral nutrition, previous renal replacement therapy, abdominal surgery within the last 7 days, and previous antimicrobial therapy for more than 48 h) [68••]. Overall, 339 patients were enrolled with an eventual IC prevalence of 14.2% (48/339). The primary endpoint was 28-day mortality, which occurred in 33.7% (58/172) and 30.5% (51/167) of patients in the BDG-driven and culture-driven arms, respectively (relative risk 1.10, with 95% confidence interval [CI] from 0.80 to 1.51). The median time to antifungal therapy was 1.1 days and 4.4 days in BDG-driven and culture-driven arms, respectively [68••]. In an exploratory subgroup analysis of the previously discussed INTENSE RCT, critically ill patients with intra-abdominal infection and positive serum BDG were more likely to have a confirmed IC than patients with negative serum BDG (odds ratio [OR] 3.66, with 95% confidence interval from 1.01 to 13.29) [50]. However, this was a secondary, exploratory, observational analysis of the data and patients were not randomized to receive antifungals based on BDG results [50]. In the EMPIRICUS RCT, the observed numerically favorable but non-statistically significant impact of micafungin on invasive fungal infection-free survival at day 28 in patients with positive serum BDG (HR 1.41 with placebo as reference, 95% CI from 0.85 to 2.33) comes from a subgroup analysis, and also deserves further investigation [49].
Finally, non-culture-based tests have also been studied for early antifungal discontinuation due to their high NPV, with RCTs suggesting a favorable effect on safely reducing early antifungal therapy duration [69, 70]. Nonetheless, some caution is needed with regard to serum BDG when extrapolating these results to settings with a high relative frequency of C. parapsilosis and/or C. auris as causative agents of IC, due to possible reduced sensitivity [61,62,63].
Pre-emptive Early Antifungal Therapy
The use of a pre-emptive approach to antifungal therapy for IC in non-neutropenic critically ill patients is currently not supported, considering the low PPV of non-culture-based tests for screening purposes in this population, although it should be highlighted that high certainty evidence in this area is currently lacking. Observational studies have provided mixed results, showing on the one hand a possible reduction in the incidence of IC, and on the other hand a possibly high cost in terms of unnecessary antifungal treatment [71,72,73,74]. Evidence from RCTs is also limited. In an RCT conducted in 64 critically ill patients requiring at least 3 days of ICU stay, pre-emptive antifungal therapy with anidulafungin based on results of twice weekly serum BDG screening was compared to empirical therapy (based on physicians’ judgment in patients blinded to serum BDG screening results) [75]. Overall, 21/45 and 5/17 evaluable patients received antifungal therapy in the pre-emptive therapy and empirical therapy arms, respectively. With the important limitation that the study was not designed or sufficiently powered to assess relevant clinical outcomes, survival at ICU discharge, length of ICU stay, proportion of febrile ICU days, and days on broad spectrum antibiotics were similar in the pre-emptive anidulafungin therapy and empirical therapy arms (85% vs. 81%, median 19 vs. 15 days, 8% vs. 12%, and median 3 vs. 4 days, respectively) [75].
Conclusions
Empirical antifungal therapy is always considered at the bedside of critically ill patients with risk factors for IC, owing to the reported increased mortality associated with delays in the start of appropriate antifungal therapy in patients with IC [8,9,10,11,12, 76]. Despite the presence of many well-designed observational studies evaluating empirical antifungal therapy in ICU patients [77,78,79,80,81,82,83,84], in the present narrative review, we decided to focus on results from RCTs, aiming to summarize those results with the highest level of evidence and to better identify current gray areas still deserving further investigation. In this regard, the first consideration to be made is that, while the direction of effect in RCTs exploring efficacy of empirical therapy in ICU patients with suspected IC was most frequently toward a favorable impact of empirical therapy, no formal demonstration of superiority was observed. This latter result stands in contrast to the increased mortality reported from observational studies in case of delayed antifungal therapy in patients with IC, suggesting, in our opinion, that further research is still necessary to better delineate the precise subgroup of ICU patients with suspected IC that would benefit from early antifungal therapy (either early empirical based on risk scores or diagnostic-driven, or a combination of both). In support of this hypothesis, it should be noted that a wide heterogeneity is present regarding the definition of IC risk in RCTs exploring the efficacy of empirical antifungal therapy in ICU patients with suspected IC, a fact that may confound comparison of findings. Furthermore, the prevalence of confirmed IC was low in the enrolled study populations, which raises the possibility of failing to detect a true effect (type 2 error) due to the predominance of patients without IC enrolled in studies. In our opinion, all of this may suggest that a more tailored identification of patients at risk of IC could both improve the outcomes of critically ill patients with IC who receive early antifungal therapy and promote antifungal stewardship. A possible solution to restrict early antifungal therapy to selected subgroups at risk is by using a diagnostic-driven early antifungal therapy approach. The most explored non-culture-based test against this backdrop is serum beta-D-glucan. Results of currently available RCTs show a trend toward a favorable effect of this approach, but are inadequately powered to draw definitive conclusions. In our opinion, further study is still needed to identify both the subgroup of patients that could benefit the most from a diagnostic-driven approach and the best non-culture-based test (or a combination of tests) to be used for this purpose. For example, we recently explored the impact of a machine learning approach to combine the diagnostic performance for IC of serum BDG and procalcitonin with an explainable machine learning prediction of IC based on other nonspecific laboratory results learned on a training set of thousands of patients [85•]. We found a trend toward improved diagnostic performance for IC, but the findings were not statistically significant. Further steps will be to explore the use of other machine learning algorithms to capture more complex relationships across features, and to explore the addition of automatically extracted clinical variables from electronic medical charts to incorporate them in machine learning-based development of early diagnostic tools based on commonly collected laboratory and clinical variables in everyday clinical practice [85•].
While waiting for further dedicated research, in clinical practice empirical antifungal therapy could be considered in critically ill patients with consistent signs and symptoms and a probability of IC > 20–25% based on risk models, or in critically ill patients with septic shock and multiorgan failure, according to expert recommendations from ESICM/ESCMID [56]. Finally, an important note to be made is that part of the heterogeneity in current research on this topic may be related to the lack of a standardized definition of IC (especially non-candidemic) in non-neutropenic critically ill patients, hampering comparison of research findings. To this end, an effort to improve comparability of research studies on IC in ICU, including RCT evaluating the impact and role of any kind of early antifungal therapy, is currently ongoing through the FUNDICU project [53•, 86].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. https://doi.org/10.1038/nrdp.2018.26.
Bassetti M, Azoulay E, Kullberg BJ, Ruhnke M, Shoham S, Vazquez J, et al. EORTC/MSGERC Definitions of Invasive Fungal Diseases: Summary of Activities of the Intensive Care Unit Working Group. Clin Infect Dis. 2021;72(2):S121–7. https://doi.org/10.1093/cid/ciaa1751. Comprehensive discussion of the difficulty of achieving a consensus definition for invasive fungals diseases, including deep-seated candidiasis, in critically ill patients.
Poissy J, Damonti L, Bignon A, Khanna N, Von Kietzell M, Boggian K, et al. Risk factors for candidemia: a prospective matched case-control study. Crit Care. 2020;24(1):109. https://doi.org/10.1186/s13054-020-2766-1.
Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5(1):161–9. https://doi.org/10.4161/viru.26187.
Kett DH, Azoulay E, Echeverria PM, Vincent JL. Extended prevalence of infection in ICUSGoI. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med. 2011;39(4):665–70. https://doi.org/10.1097/CCM.0b013e318206c1ca.
Bassetti M, Righi E, Ansaldi F, Merelli M, Scarparo C, Antonelli M, et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med. 2015;41(9):1601–10. https://doi.org/10.1007/s00134-015-3866-2.
Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–56. https://doi.org/10.1056/NEJMra1315399.
Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–5. https://doi.org/10.1128/AAC.49.9.3640-3645.2005.
Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med. 2008;36(11):2967–72. https://doi.org/10.1097/CCM.0b013e31818b3477.
Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54(12):1739–46. https://doi.org/10.1093/cid/cis305.
Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, Perez Ruiz, de Pipaon M, Hernandez-Caballero C, Lepe-Jimenez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother. 2013;68(1):206–13. https://doi.org/10.1093/jac/dks347.
Bassetti M, Righi E, Ansaldi F, Merelli M, Trucchi C, De Pascale G, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014;40(6):839–45. https://doi.org/10.1007/s00134-014-3310-z.
Bassetti M, Giacobbe DR, Vena A, Trucchi C, Ansaldi F, Antonelli M, et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care. 2019;23(1):219. https://doi.org/10.1186/s13054-019-2497-3.
Paiva JA, Pereira JM, Tabah A, Mikstacki A, de Carvalho FB, Koulenti D, et al. Characteristics and risk factors for 28-day mortality of hospital acquired fungemias in ICUs: data from the EUROBACT study. Crit Care. 2016;20:53. https://doi.org/10.1186/s13054-016-1229-1.
Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997–2016. Open Forum Infect Dis. 2019;6(Suppl 1):S79–94. https://doi.org/10.1093/ofid/ofy358.
Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73(suppl_1):i4–13. https://doi.org/10.1093/jac/dkx444.
Giacobbe DR, Maraolo AE, Simeon V, Magne F, Pace MC, Gentile I, et al. Changes in the relative prevalence of candidaemia due to non-albicans Candida species in adult in-patients: a systematic review, meta-analysis and meta-regression. Mycoses. 2020;63(4):334–42. https://doi.org/10.1111/myc.13054.
Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild M, Bohlius J, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25(10):1200–12. https://doi.org/10.1016/j.cmi.2019.04.024.
Tabah A, Buetti N, Staiquly Q, Ruckly S, Akova M, Aslan AT, et al. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. 2023;49(2):178–90. https://doi.org/10.1007/s00134-022-06944-2. Recent udpate of the EUROBACT study on epidemiology and outcome of bloodstream infections, including candidemia, in critically ill patients worldwide.
Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–87. https://doi.org/10.1001/jama.2020.2717.
Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, et al. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, 2008–2014. Open Forum Infect Dis. 2015;2(4):163. https://doi.org/10.1093/ofid/ofv163.
Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56(12):1724–32. https://doi.org/10.1093/cid/cit136.
Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, et al. Role of FKS Mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother. 2014;58(8):4690–6. https://doi.org/10.1128/AAC.03255-14.
Briano F, Magnasco L, Sepulcri C, Dettori S, Dentone C, Mikulska M, et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect Dis Ther. 2022;11(3):1149–60. https://doi.org/10.1007/s40121-022-00625-9. Recent original article detailing the risk of Candida auris candidemia in colonized patients in intensive care units.
Cortegiani A, Misseri G, Chowdhary A. What’s new on emerging resistant Candida species. Intensive Care Med. 2019;45(4):512–5. https://doi.org/10.1007/s00134-018-5363-x.
Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, et al. Candida auris isolates resistant to three classes of antifungal medications - New York, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(1):6–9. https://doi.org/10.15585/mmwr.mm6901a2.
Giacobbe DR, Mikulska M, Vena A, Di Pilato V, Magnasco L, Marchese A, Bassetti M. Challenges in the diagnosis and treatment of candidemia due to multidrug-resistant Candida auris. Front Fungal Biol. 2023;4:1061150. https://doi.org/10.3389/ffunb.2023.1061150.
Codda G, Willison E, Magnasco L, Morici P, Giacobbe DR, Mencacci A, et al. In vivo evolution to echinocandin resistance and increasing clonal heterogeneity in Candida auris during a difficult-to-control hospital outbreak, Italy, 2019 to 2022. Euro Surveill. 2023;28:14. https://doi.org/10.2807/1560-7917.ES.2023.28.14.2300161.
Schelenz S, Barnes RA, Barton RC, Cleverley JR, Lucas SB, Kibbler CC, et al. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect Dis. 2015;15(4):461–74. https://doi.org/10.1016/S1473-3099(15)70006-X.
Clancy CJ, Nguyen MH. Non-culture diagnostics for invasive candidiasis: promise and unintended consequences. J Fungi (Basel, Switzerland). 2018;4:1. https://doi.org/10.3390/jof4010027.
Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284–92. https://doi.org/10.1093/cid/cit006.
Muskett H, Shahin J, Eyres G, Harvey S, Rowan K, Harrison D. Risk factors for invasive fungal disease in critically ill adult patients: a systematic review. Crit Care. 2011;15(6):R287. https://doi.org/10.1186/cc10574.
Jameran AS, Cheah SK, Tzar MN, Musthafa QA, Low HJ, Maaya M, Abdul RR. An approach to develop clinical prediction rule for candidemia in critically ill patients: a retrospective observational study. J Crit Care. 2021;65:216–20. https://doi.org/10.1016/j.jcrc.2021.06.018.
Calandra T, Roberts JA, Antonelli M, Bassetti M, Vincent JL. Diagnosis and management of invasive candidiasis in the ICU: an updated approach to an old enemy. Crit Care. 2016;20(1):125. https://doi.org/10.1186/s13054-016-1313-6.
Thomas-Ruddel DO, Schlattmann P, Pletz M, Kurzai O, Bloos F. Risk factors for invasive candida infection in critically ill patients: a systematic review and meta-analysis. Chest. 2022;161(2):345–55. https://doi.org/10.1016/j.chest.2021.08.081.
Leroy G, Lambiotte F, Thevenin D, Lemaire C, Parmentier E, Devos P, Leroy O. Evaluation of “Candida score” in critically ill patients: a prospective, multicenter, observational, cohort study. Ann Intensive Care. 2011;1(1):50. https://doi.org/10.1186/2110-5820-1-50.
Hermsen ED, Zapapas MK, Maiefski M, Rupp ME, Freifeld AG, Kalil AC. Validation and comparison of clinical prediction rules for invasive candidiasis in intensive care unit patients: a matched case-control study. Crit Care. 2011;15(4):R198. https://doi.org/10.1186/cc10366.
Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220(6):751–8. https://doi.org/10.1097/00000658-199412000-00008.
Paphitou NI, Ostrosky-Zeichner L, Rex JH. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med Mycol. 2005;43(3):235–43. https://doi.org/10.1080/13693780410001731619.
Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2007;26(4):271–6. https://doi.org/10.1007/s10096-007-0270-z.
Leon C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34(3):730–7. https://doi.org/10.1097/01.CCM.0000202208.37364.7D.
Ostrosky-Zeichner L. Clinical prediction rules for invasive candidiasis in the ICU: ready for prime time? Crit Care. 2011;15(5):189. https://doi.org/10.1186/cc10422.
Michalopoulos AS, Geroulanos S, Mentzelopoulos SD. Determinants of candidemia and candidemia-related death in cardiothoracic ICU patients. Chest. 2003;124(6):2244–55. https://doi.org/10.1378/chest.124.6.2244.
Bassetti M, Giacobbe DR, Vena A, Wolff M. Diagnosis and treatment of candidemia in the intensive care unit. Semin Respir Crit Care Med. 2019;40(4):524–39. https://doi.org/10.1055/s-0039-1693704.
Solomkin JS, Flohr AM, Simmons RL. Indications for therapy for fungemia in postoperative patients. Arch Surg. 1982;117(10):1272–5. https://doi.org/10.1001/archsurg.1982.01380340008003.
Bernhardt HE, Orlando JC, Benfield JR, Hirose FM, Foos RY. Disseminated candidiasis in surgical patients. Surg Gynecol Obstet. 1972;134(5):819–25.
Guillamet CV, Vazquez R, Micek ST, Ursu O, Kollef M. Development and validation of a clinical prediction rule for candidemia in hospitalized patients with severe sepsis and septic shock. J Crit Care. 2015;30(4):715–20. https://doi.org/10.1016/j.jcrc.2015.03.010.
Playford EG, Lipman J, Jones M, Lau AF, Kabir M, Chen SC, et al. Problematic dichotomization of risk for intensive care unit (ICU)-acquired invasive candidiasis: results using a risk-predictive model to categorize 3 levels of risk from a multicenter prospective cohort of Australian ICU patients. Clin Infect Dis. 2016;63(11):1463–9. https://doi.org/10.1093/cid/ciw610.
Timsit JF, Azoulay E, Schwebel C, Charles PE, Cornet M, Souweine B, et al. Empirical Micafungin treatment and survival without invasive fungal infection in adults with ICU-acquired sepsis, Candida colonization, and multiple organ failure: the EMPIRICUS randomized clinical trial. JAMA. 2016;316(15):1555–64. https://doi.org/10.1001/jama.2016.14655.
Knitsch W, Vincent JL, Utzolino S, Francois B, Dinya T, Dimopoulos G, et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis. 2015;61(11):1671–8. https://doi.org/10.1093/cid/civ707.
Schuster MG, Edwards JE Jr, Sobel JD, Darouiche RO, Karchmer AW, Hadley S, et al. Empirical fluconazole versus placebo for intensive care unit patients: a randomized trial. Ann Intern Med. 2008;149(2):83–90. https://doi.org/10.7326/0003-4819-149-2-200807150-00004.
Johnson MD, Lewis RE, Dodds Ashley ES, Ostrosky-Zeichner L, Zaoutis T, Thompson GR, et al. Core recommendations for antifungal stewardship: a statement of the Mycoses Study Group Education and Research Consortium. J Infect Dis. 2020;222(Suppl 3):S175–98. https://doi.org/10.1093/infdis/jiaa394.
Giacobbe DR, Asperges E, Cortegiani A, Grecchi C, Rebuffi C, Zuccaro V, et al. Performance of existing clinical scores and laboratory tests for the diagnosis of invasive candidiasis in critically ill, nonneutropenic, adult patients: A systematic review with qualitative evidence synthesis. Mycoses. 2022;65(12):1073–111. https://doi.org/10.1111/myc.13515. Systematic review within the FUNDICU project and dedicated to an updated assessment of the diagnostic performance for IC in critically ill patients of existing clinical scores (that guide empirical antifungal therapy) and laboratory tests (that guide diagnostic-driven early antifungal therapy).
Posteraro B, Tumbarello M, De Pascale G, Liberto E, Vallecoccia MS, De Carolis E, et al. (1,3)-beta-d-Glucan-based antifungal treatment in critically ill adults at high risk of candidaemia: an observational study. J Antimicrob Chemother. 2016;71(8):2262–9. https://doi.org/10.1093/jac/dkw112.
He S, Hang JP, Zhang L, Wang F, Zhang DC, Gong FH. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-beta-D-glucan for invasive fungal infection: focus on cutoff levels. J Microbiol Immunol Infect. 2015;48(4):351–61. https://doi.org/10.1016/j.jmii.2014.06.009.
Martin-Loeches I, Antonelli M, Cuenca-Estrella M, Dimopoulos G, Einav S, De Waele JJ, et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45(6):789–805. https://doi.org/10.1007/s00134-019-05599-w.
Leon C, Ruiz-Santana S, Saavedra P, Castro C, Loza A, Zakariya I, et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Crit Care. 2016;20(1):149. https://doi.org/10.1186/s13054-016-1324-3.
Giacobbe DR, Mikulska M, Tumbarello M, Furfaro E, Spadaro M, Losito AR, et al. Combined use of serum (1,3)-beta-D-glucan and procalcitonin for the early differential diagnosis between candidaemia and bacteraemia in intensive care units. Crit Care. 2017;21(1):176. https://doi.org/10.1186/s13054-017-1763-5.
Giacobbe DR, Signori A, Tumbarello M, Ungaro R, Sarteschi G, Furfaro E, et al. Desirability of outcome ranking (DOOR) for comparing diagnostic tools and early therapeutic choices in patients with suspected candidemia. Eur J Clin Microbiol Infect Dis. 2019;38(2):413–7. https://doi.org/10.1007/s10096-018-3441-1.
Giacobbe DR, Signori A. Interpreting desirability of outcome ranking (DOOR) analyses in observational studies in infectious diseases: caution still needed. Eur J Clin Microbiol Infect Dis. 2019;38(10):1985–6. https://doi.org/10.1007/s10096-019-03612-0.
Mikulska M, Magnasco L, Signori A, Sepulcri C, Dettori S, Tutino S, et al. Sensitivity of serum Beta-D-Glucan in candidemia according to Candida species epidemiology in critically ill patients admitted to the intensive care unit. J Fungi (Basel, Switzerland). 2022;8:9. https://doi.org/10.3390/jof8090921.
Mikulska M, Giacobbe DR, Furfaro E, Mesini A, Marchese A, Del Bono V, Viscoli C. Lower sensitivity of serum (1,3)-beta-d-glucan for the diagnosis of candidaemia due to Candida parapsilosis. Clin Microbiol Infect. 2016;22(7):6465–8. https://doi.org/10.1016/j.cmi.2016.05.020.
Chibabhai V, Fadana V, Bosman N, Nana T. Comparative sensitivity of 1,3 beta-D-glucan for common causes of candidaemia in South Africa. Mycoses. 2019;62(11):1023–8. https://doi.org/10.1111/myc.12982.
Novy E, Riviere J, Nguyen M, Arfeuille G, Louis G, Bouhemad B, et al. Combination of serum and peritoneal 1.3-beta-D-glucan can rule out intra-abdominal candidiasis in surgical critically ill patients: a multicenter prospective study. Crit Care. 2023;27(1):470. https://doi.org/10.1186/s13054-023-04761-7. Recent orginal article highlighting the high NPV of the combination of serum and peritoneal BDG for excluding IAC in critically ill patients.
O’Donnell M, Shields RK, Marini RV, Groetzinger LM, Potoski BA, Falcione BA, et al. Stewardship-guided T2Candida testing shortens time to antifungal treatment and reduces antifungal usage among medical intensive care unit patients with septic shock. Open Forum Infect Dis. 2023;10(11):ofad538. https://doi.org/10.1093/ofid/ofad538.
Lamoth F, Clancy CJ, Tissot F, Squires K, Eggimann P, Fluckiger U, et al. Performance of the T2Candida panel for the diagnosis of intra-abdominal candidiasis. Open Forum Infect Dis. 2020;7(3):ofaa075. https://doi.org/10.1093/ofid/ofaa075.
Arendrup MC, Andersen JS, Holten MK, Krarup KB, Reiter N, Schierbeck J, Helleberg M. Diagnostic performance of T2Candida among ICU patients with risk factors for invasive candidiasis. Open Forum Infect Dis. 2019;6(5):ofz136. https://doi.org/10.1093/ofid/ofz136.
Bloos F, Held J, Kluge S, Simon P, Kogelmann K, de Heer G, et al. (1 --> 3)-beta-D-Glucan-guided antifungal therapy in adults with sepsis: the CandiSep randomized clinical trial. Intensive Care Med. 2022;48(7):865–75. https://doi.org/10.1007/s00134-022-06733-x. Recent results of the CandiSep RCT exploring the role of serum BDG-guided early antifungal therapy in critically ill patients with sepsis.
Rouze A, Loridant S, Poissy J, Dervaux B, Sendid B, Cornu M, et al. Biomarker-based strategy for early discontinuation of empirical antifungal treatment in critically ill patients: a randomized controlled trial. Intensive Care Med. 2017;43(11):1668–77. https://doi.org/10.1007/s00134-017-4932-8.
De Pascale G, Posteraro B, D’Arrigo S, Spinazzola G, Gaspari R, Bello G, et al. (1,3)-Beta-D-Glucan-based empirical antifungal interruption in suspected invasive candidiasis: a randomized trial. Crit Care. 2020;24(1):550. https://doi.org/10.1186/s13054-020-03265-y.
Wissing H, Ballus J, Bingold TM, Nocea G, Krobot KJ, Kaskel P, et al. Intensive care unit-related fluconazole use in Spain and Germany: patient characteristics and outcomes of a prospective multicenter longitudinal observational study. Infect Drug Resist. 2013;6:15–25. https://doi.org/10.2147/IDR.S38945.
Piarroux R, Grenouillet F, Balvay P, Tran V, Blasco G, Millon L, Boillot A. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med. 2004;32(12):2443–9. https://doi.org/10.1097/01.ccm.0000147726.62304.7f.
Pang YK, Ip M, You JHS. Potential clinical and economic outcomes of active beta-D-glucan surveillance with preemptive therapy for invasive candidiasis at intensive care units: a decision model analysis. Eur J Clin Microbiol Infect Dis. 2017;36(1):187–94. https://doi.org/10.1007/s10096-016-2796-4.
Azoulay E, Dupont H, Tabah A, Lortholary O, Stahl JP, Francais A, et al. Systemic antifungal therapy in critically ill patients without invasive fungal infection*. Crit Care Med. 2012;40(3):813–22. https://doi.org/10.1097/CCM.0b013e318236f297.
Hanson KE, Pfeiffer CD, Lease ED, Balch AH, Zaas AK, Perfect JR, Alexander BD. Beta-D-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study. PLoS ONE. 2012;7(8):e42282. https://doi.org/10.1371/journal.pone.0042282.
Hoenigl M, Salmanton-Garcia J, Egger M, Gangneux JP, Bicanic T, Arikan-Akdagli S, et al. Guideline adherence and survival of patients with candidaemia in Europe: results from the ECMM Candida III multinational European observational cohort study. Lancet Infect Dis. 2023;23(6):751–61. https://doi.org/10.1016/S1473-3099(22)00872-6.
Tang Y, Hu W, Jiang S, Xie M, Zhu W, Zhang L, et al. Effect of empirical antifungal treatment on mortality in non-neutropenic critically ill patients: a propensity-matched retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2022;41(12):1421–32. https://doi.org/10.1007/s10096-022-04507-3.
Montravers P, Perrigault PF, Timsit JF, Mira JP, Lortholary O, Leroy O, et al. Antifungal therapy for patients with proven or suspected Candida peritonitis: Amarcand2, a prospective cohort study in French intensive care units. Clin Microbiol Infect. 2017;23(2):117 e1-e8. https://doi.org/10.1016/j.cmi.2016.10.001.
Micek ST, Arnold H, Juang P, Hampton N, McKenzie M, Scolarici M, Kollef M. Effects of empiric antifungal therapy for septic shock on time to appropriate therapy for Candida infection: a pilot study. Clin Ther. 2014;36(9):1226–32. https://doi.org/10.1016/j.clinthera.2014.06.028.
Leroy O, Bailly S, Gangneux JP, Mira JP, Devos P, Dupont H, et al. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care. 2016;6(1):2. https://doi.org/10.1186/s13613-015-0103-7.
Hasan MJ, Neelotpol S, Rabbani R. Early empirical anidulafungin reduces the prevalence of invasive candidiasis in critically Ill patients: a case-control study. J Crit Care Med (Targu Mures). 2022;8(2):89–99. https://doi.org/10.2478/jccm-2022-0006.
Gonzalez de Molina FJ, Leon C, Ruiz-Santana S, Saavedra P. Group CIS Assessment of candidemia-attributable mortality in critically ill patients using propensity score matching analysis. Crit Care. 2012;16(3):R150. https://doi.org/10.1186/cc11388.
Bruyere R, Quenot JP, Prin S, Dalle F, Vigneron C, Aho S, et al. Empirical antifungal therapy with an echinocandin in critically-ill patients: prospective evaluation of a pragmatic Candida score-based strategy in one medical ICU. BMC Infect Dis. 2014;14:385. https://doi.org/10.1186/1471-2334-14-385.
Bailly S, Bouadma L, Azoulay E, Orgeas MG, Adrie C, Souweine B, et al. Failure of empirical systemic antifungal therapy in mechanically ventilated critically ill patients. Am J Respir Crit Care Med. 2015;191(10):1139–46. https://doi.org/10.1164/rccm.201409-1701OC.
Giacobbe DR, Marelli C, Mora S, Guastavino S, Russo C, Brucci G, et al. Early diagnosis of candidemia with explainable machine learning on automatically extracted laboratory and microbiological data: results of the AUTO-CAND project. Ann Med. 2023;55(2):2285454. https://doi.org/10.1080/07853890.2023.2285454. Recent results of a different approach to biomarker-guided early detection of candidemia (for diagnostic-driven early antifungal therapy) by means on machine learning techniques on automatically extracted microbiological and laboratory data.
Bassetti M, Scudeller L, Giacobbe DR, Lamoth F, Righi E, Zuccaro V, et al. Developing definitions for invasive fungal diseases in critically ill adult patients in intensive care units. Protocol of the FUNgal infections Definitions in ICU patients (FUNDICU) project. Mycoses. 2019;62(4):310–9. https://doi.org/10.1111/myc.12869.
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization: D.R.G., M.B.; writing—original draft preparation, C.B., D.R.G.; writing—review and editing, C.B., D.R.G., A.V., M.B.; supervision: D.R.G., A.V., M.B.
Corresponding author
Ethics declarations
Conflict of Interest
Outside the submitted work, M.B. has received funding for scientific advisory boards, travel, and speaker honoraria from Angelini, Astellas, BioMérieux, Cidara, Gilead, Menarini, MSD, Pfizer, Shionogi, Tetraphase, Nabriva. Outside the submitted work, D.R.G. reports investigator-initiated grants from Pfizer, Shionogi, BioMérieux, and Gilead Italia, and speaker/advisor fees from Menarini, Pfizer, and Tillotts Pharma. The other authors have no conflicts of interests to disclose.
Human and Animals Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bartalucci, C., Giacobbe, D.R., Vena, A. et al. Empirical Therapy for Invasive Candidiasis in Critically Ill Patients. Curr Fungal Infect Rep (2024). https://doi.org/10.1007/s12281-024-00489-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12281-024-00489-1