Abstract
Dengue virus (DENV), belonging to the family Flaviviridae, is the causative agent of dengue and comprises four serotypes. A second heterologous DENV infection is a critical risk factor for severe dengue, and no effective vaccine is available to prevent infection by all four DENV serotypes. Recombinant DENV vaccines are primarily based on the envelope proteins, prM and E. The E protein and its envelope domain III (EDIII) have been investigated as candidate antigens (Ags) for recombinant subunit vaccines. However, most EDIII-based Ags are monomers that do not display the cognate antigenic structure of E protein, which is essential for induction of virus-neutralizing immunity. Here, we developed recombinant DENV-2 envelope domain (r2ED) protein as an Ag that mimics the quaternary structure of E protein on the DENV surface. We confirmed that r2ED retained the conformational epitope displayed at the E-dimer interface, which reportedly exhibits broad virus-neutralizing capacity, without displaying the fusion loop epitope that causes antibody (Ab)-dependent enhancement. Furthermore, compared with EDIII alone, r2ED elicited stronger Ag-specific and cross-reactive neutralizing Ab and T cell-mediated immune responses in mice. This Ag-specific immunity was maintained at an elevated level 6 months after the last immunization, suggesting sustained Ag-specific immune memory. Taken together, these observations suggest that r2ED could be used to develop an improved subunit vaccine capable of inducing a broadly cross-reactive and long-lasting immune response against DENV infection.
Similar content being viewed by others
Introduction
Dengue is a mosquito-borne viral disease caused by a virus that belongs to the Flaviviridae family. There are four closely related but distinct serotypes of the dengue virus (DENV; DENV-1, -2, -3, and -4) that cause dengue. Although all four DENV serotypes exhibit year-round circulation, DENV-1 and DENV-2 are the main circulating serotypes (Ministry of Health, 2012). DENV-1 and DENV-2 are also the main infecting serotypes in dengue-confirmed patients (DENV-1, 22.0%; DENV-2, 57.1%; DENV-3, 17.1%; DENV-4, 3.8%) (Yung et al., 2015). Compared with other serotypes, secondary infection with DENV-2 is more likely to result in severe dengue (Balmaseda et al., 2006; Fried et al., 2010). The global incidence of dengue is estimated to be 390 million per year, among which 96 million infections are clinically severe (Bhatt et al., 2013). Moreover, the geographical range of dengue risk is expected to expand because of climate change (Messina et al., 2019). However, supportive care remains the sole therapy for dengue.
Multiple vaccine formulations for DENV, including live-attenuated vaccine, inactivated virus vaccine, subunit vaccine, and DNA vaccine, are at different stages of development (Park et al., 2022; Pinheiro-Michelsen et al., 2020). The most important aspect of dengue vaccine development, particularly with respect to tetravalent vaccines, is that the immune responses to all four DENV serotypes must be balanced. The only licensed vaccine, Dengvaxia®, reportedly results in unbalanced antibody (Ab) responses to nondominant serotypes because of interserotype interference, which could lead to severe disease upon infection with DENV (Halstead, 2017). Live-attenuated dengue vaccines can deliver a set of antigens (Ags) that encompasses all four DENV serotypes and provide long-term protective immunity. However, the immunodominance caused by dominant serotype-biased replication causes distortion of both Ab and T-cell responses, resulting in a suboptimal response to at least one serotype (Rothman, 2011). Most live-attenuated dengue vaccines are based on chimeric or attenuated live viruses containing the envelope proteins, pre-membrane (prM) and envelope (E), which are considered immunogens and potential targets for protective immune response induction (Beltramello et al., 2010; Lai et al., 2008). Live-attenuated dengue vaccines predominantly elicit Abs against prM and the fusion loop epitope (FLE) of envelope domain II (EDII) (Shukla et al., 2020). These Abs are generally cross-reactive among DENV serotypes and have a high risk of promoting Ab-dependent enhancement (ADE), a phenomenon in which suboptimal neutralizing or non-neutralizing cross-reactive Abs bind to virus and thus facilitate Fc receptor-mediated enhanced virus entry into host cells; this leads to an increase in cellular viral load (Shukla et al., 2020).
Various candidates for DENV subunit vaccines based on recombinant Ags have been developed. Notably, the tetravalent vaccine candidate is based on the EDIIIs of all four DENVs fused using flexible linkers in a single translational reading frame. Unlike EDI and EDII, which elicit mostly cross-reactive and weakly or non-neutralizing Abs, EDIII elicits effective serotype-specific neutralizing Abs with low potential for ADE (Shukla et al., 2020). However, EDIII has a potential limitation as an immunogen, in that it elicits non-neutralizing Abs for inaccessible Ag sites such as the AB loop, which is highly conserved among DENV serotypes within the pre-fusion E dimer of the virion (Li et al., 2013; Midgley et al., 2012). The AB loop (314–317 aa) is present in the buried surface area of EDIII protein, and the cross-reactive Abs recognize residues of strands A and E and the D-Dx loop as well as AB loop (Midgley et al., 2012). Furthermore, the immune response elicited by subunit vaccines may not be maintained for an extended duration, compared with the response elicited by live-attenuated vaccines (Swaminathan & Khanna, 2019). The results of recent studies suggest that antigenic epitopes presented on the quaternary structure of the intact virion can be used as immunogens for subunit vaccines, with the expectation that they will minimize inefficient Ab responses to DENV (Beltramello et al., 2010; Dejnirattisai et al., 2010; Priyamvada et al., 2016). Several human monoclonal Abs (mAbs) have been isolated with the ability to recognize the quaternary antigenic structure and the capacity to maintain strong neutralizing activity and broad reactivity across multiple DENV serotypes (Dejnirattisai et al., 2015; Gallichotte et al., 2015). Additionally, envelope-dimer epitopes (EDEs) were identified as the major quaternary antigenic epitopes recognized by these Abs (Dejnirattisai et al., 2015). EDEs can be formed by the E-dimer interface, which consists of residues on EDIII and EDII of two different monomers within a single dimer and overlaps with the FLE, thus limiting FLE display to avoid the poor neutralizing and infection-enhancing characteristics of FLE (Park et al., 2020). Compared with E monomer, E dimer-based EDEs elicit high levels of serotype-specific immune responses; because of the preserved EDE region, E dimer-based EDEs also elicit DENV cross-reactive Abs that neutralize all four DENV serotypes (Barba-Spaeth et al., 2016; Fernandez et al., 2017; Rouvinski et al., 2017; Thomas et al., 2020). Therefore, E dimer-based Ag could be an ideal vaccine candidate for the induction of highly potent and broadly neutralizing Abs, as well as the elimination of ADE risk.
In this study, we developed a recombinant Ag of the DENV-2 envelope domain (r2ED) that displays the EDE and overlaps with the FLE, thereby mimicking the antigenic structure of E protein on the surface of DENV. We then compared the abilities of r2ED and EDIII to stimulate an Ag-specific immune response and to sustain immune memory response in vivo.
Materials and Methods
Experimental Animals and Materials
Six-week-old female BALB/c mice were purchased from the Koatech Laboratory Animal Center and housed under specific pathogen-free conditions with water and food provided ad libitum. Animal experiments were approved and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Jeonbuk National University (Approval No. CBNU 2020–018). DENVs were obtained from the Korea Bank for Pathogenic Viruses (Korea University). Unless otherwise specified, the chemicals and laboratory equipment used in this study were obtained from Sigma Chemical Co. and SPL Life Sciences, respectively.
Cell Culture
Vero E6 cells (KCLB No. 21587) obtained from the Korean Cell Line Bank (Seoul National University) were used to propagate DENVs and to conduct viral neutralization assays. Vero E6 cells were cultured in Dulbecco’s modified Eagle’s medium (Welgene) supplemented with 10% fetal bovine serum (FBS; Gibco).
Recombinant Protein Production
Gene cloning, gene expression, and the purification of r2ED (Fig. 1A) and the EDIII domain were performed as described previously, with minor modifications (Park et al., 2020). To obtain the genes encoding EDIII (297–394 aa, GenBank: KP406804.1) and partial EDII and EDI region (50–192 aa), cDNA was prepared from DENV-2-infected cells. Polymerase chain reaction (PCR) was performed to amplify the EDIII gene from DENV-2 cDNA using the forward primer 5′-CTC GAG ATG TCA TAC TCT ATG TGT-3′ (XhoI restriction site is underlined) and the reverse primer 5′-AAG CTT CTA TTT CTT GAA CCA GTT-3′ (HindIII restriction site is underlined). Additionally, the partial EDII gene with the EDI gene at its 3′-terminus was amplified by PCR using the forward primer 5′-CAT ATG GCC AAA CAA CCC GCC ACT-3′ (NdeI restriction site is underlined) and the reverse primer 5′-CTC GAG GTC AAG GCC CGT TCT CGG-3′ (XhoI restriction site is underlined). The two amplified fragments were combined to generate a partial EDII–EDI–EDIII recombinant gene, which was then cloned into the pColdII Escherichia coli expression vector (TaKaRa Bio). The amplified EDIII gene was also cloned into the pColdII vector. Recombinant proteins were purified by Ni–NTA Superflow (Qiagen) for proteins with an N-terminal His tag, in accordance with the manufacturer’s instructions. Recombinant EDIII and r2ED Ags were > 95% pure, and the final endotoxin content was < 0.5 EU/μg of Ags as measured by the LAL chromogenic endotoxin quantitation kit (Thermo Fisher Scientific). The sizes and identities of recombinant proteins were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by western blotting and enzyme-linked immunosorbent assay (ELISA) with anti-DENV EDE1 (Creative Biolabs) and Penta-His (Qiagen) Abs.
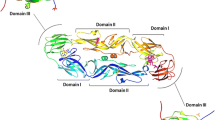
Characterization of recombinant DENV-2 envelope domain (r2ED) protein. A Predicted structure of DENV E protein dimer and design of r2ED. Domains I (EDI), II (EDII), and III (EDIII) are represented by red, yellow, and blue bars, respectively. Stem and transmembrane (TM) regions are represented in black and white, respectively. The FLE is buried at the dimer interface and the EDE is overlapped with the FLE. Structures were generated using UCSF Chimera. B Purified recombinant proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blotting with Penta-His Ab. C Binding of anti-DENV EDE1 mAb to EDIII and r2ED was determined by direct ELISA under nonreducing and reducing conditions
Mouse Immunization and Sample Collection
BALB/c mice were immunized subcutaneously at the base of the tail and intramuscularly in the hind leg with 10 μg/mouse of each recombinant protein, which had been dissolved in 30 μl of phosphate-buffered saline (PBS) and emulsified with an equal volume of Freund’s complete adjuvant; at 10 days after the first immunization, mice were boosted once with the same immunogen, emulsified with Freund’s incomplete adjuvant. Control mice were immunized with an identically prepared inoculum that contained only PBS (no recombinant protein). Sera were collected 3 days after booster immunization to assess the Ag-specific Ab response. The spleen was collected 4 days after booster immunization to monitor Ag-specific T-cell responses. To investigate the effects of Ag re-exposure, immunized mice were boosted 6 months after the last immunization by subcutaneous injection of each recombinant protein; serum and spleen were then prepared to measure levels of Ag-specific immune responses, as described previously (Park et al., 2020).
ELISA
Levels of Ag-specific Ab in mouse serum samples were determined by ELISA using EDIII as a target Ag because the r2ED protein also contains the same EDIII domain. Briefly, 96-well ELISA plates (Thermo Fisher Scientific Europe) were precoated with EDIII protein (1 μg/ml) overnight at 4 °C and blocked with 5% nonfat dried milk at 37 °C for 2 h. After the addition of serially diluted serum samples to each well, the plates were incubated at 37 °C for 1 h, then washed four times with PBS containing Tween-20. Bound Abs were incubated with alkaline phosphate-conjugated anti-mouse IgG at 37 °C for 1 h, and reactions were visualized by the addition of p-nitrophenyl phosphate substrate. Color development was measured by reading the absorbance at 405 nm on an ELISA plate reader (SPECTROstar Nano; BMG Labtech).
Flow Cytometry
To assess the levels of Ag-specific T-cell responses after immunization, spleens were collected from immunized mice and splenocytes were prepared by Percoll gradient centrifugation after treatment with ammonium-chloride-potassium (ACK) lysis buffer to remove red blood cells. For T-cell restimulation experiments, splenocytes were plated at 2 × 106 cells per well in 48-well plates and incubated with 1 μg of Ag protein or phorbol myristate acetate (50 ng/ml) plus ionomycin (1 μg/ml) as a control for 20 h at 37 °C with 5% CO2. In some Ag-specific cytotoxic T lymphocyte-confirmed samples, anti-CD107a Ab was added during stimulation. To characterize the T-cell population by flow cytometry, cells were stained with the indicated fluorochrome-conjugated Abs in PBS containing 2% FBS after they had been subjected to Fc receptor blocking. The following Abs were used: anti-CD3-fluorescein isothiocyanate (130-119-758; Miltenyi Biotec), anti-CD4-peridinin-chlorophyll-protein (PerCP)-Vio® 700 (130-118-794, Miltenyi Biotec), anti-CD8-phycoerythrin (130-123-781, Miltenyi Biotec), anti-CD44-allophycocyanin (130-119-121, Miltenyi Biotec), and anti-CD107a-allophycocyanin (130-111-319, Miltenyi Biotec). To identify CD4+ helper T cells and CD8+ cytotoxic T cells, the cells were initially gated on CD3, then gated on CD4 and CD8, respectively. Samples were stained as described above, then fixed with PBS containing 1% paraformaldehyde. Flow cytometry was performed (CytoFLEX; Beckman Coulter) and data were analyzed using CytExpert software (Beckman Coulter).
For analysis of cytokine production by T cells after Ag stimulation, splenocytes were stimulated with Ag protein for 20 h; culture supernatants were then collected and immediately frozen at -80 °C. Cytokines present in pooled supernatant samples (n = 3 per group) were quantified using a BD Cytometric Bead Array Mouse Th1/Th2/Th17 cytokine kit (BD Biosciences), in accordance with the manufacturer’s instructions, and analyzed using FCAP Array software (Soft Flow).
Virus Propagation, Titration, and Neutralization
To propagate DENVs, Vero E6 cells were infected with a small volume of DENV at a multiplicity of infection of 0.1 in 5% FBS-containing culture medium. After incubation for 7–10 days at 37 °C with 5% CO2, the culture supernatants were harvested. The viruses were concentrated by centrifugation at 20,000 × g for 2 h at 4 °C, and the concentrated viruses were stored as aliquots at -80 °C (Park et al., 2020). For assessment of viral titer, Vero E6 cells were seeded at a density of 1.5 × 104 cells per well in 96-well plates, 1 day before DENV treatment. The cells were treated with two-fold serial dilutions of viral samples for 1 h at 37 °C with 5% CO2, then incubated under overlay medium (Opti-MEM with 2% FBS, antibiotics, and 1% methylcellulose) for 3 days at 37 °C with 5% CO2. Subsequently, cells were fixed with a mixture of acetone and methanol (1:1) at room temperature for 30 min, blocked with 5% nonfat milk, and incubated with anti-DENV (Santa Cruz Biotechnology) and anti-flavivirus group Ag (Merck Millipore) Abs for 2 h at room temperature. Finally, horseradish peroxidase-conjugated anti-mouse IgG Ab (Cell Signaling Technology) was added, and the clusters of infected cells or foci were identified using KPL TrueBlue™ Substrate (Seracare). The number of stained foci was used to calculate the viral titer as focus-forming units (FFU) per milliliter of suspension.
To perform focus reduction neutralization tests (FRNTs), sera obtained from mice immunized with each recombinant protein were mixed with 3 × 103 FFU of DENVs and incubated for 1 h at 37 °C. Next, each virus-serum mixture was applied to Vero E6 cells that had been seeded in 96-well plates on the previous day, then cultured for 1 h at 37 °C. Additionally, methylcellulose overlay medium was added to each sample and incubated for 3 days at 37 °C with 5% CO2. Finally, the neutralizing activity against DENVs was compared by counting the number of stained foci, as described above.
Furthermore, the neutralizing capacity of each individual serum sample was assessed by determining the viral load in DENV-infected cells through quantification of capsid (C) gene expression via quantitative real-time PCR (qRT-PCR) (Paudel et al., 2011; Thomas et al., 2020). Briefly, 100-fold diluted samples of immunized mouse serum were incubated for 1 h at room temperature with 3 × 103 FFU of DENVs, then transferred to Vero E6 cells (2.5–5 × 105 cells/well) that had been grown in 6-well plates. After cells had been incubated for 24 h, total RNA was extracted and subjected to qRT-PCR as described above using the primer set listed in Table 1 to measure the level of DENV C gene expression.
Statistical Analysis
Statistical analyses were performed using Prism 7 (GraphPad). Data are presented as means ± standard deviations (SDs). Numerical data were analyzed by two-way analysis of variance, and p < 0.05 was considered indicative of statistical significance.
Results
r2ED Mimics the DENV E-dimer Epitope in Ab Recognition
We constructed the r2ED protein Ag, which corresponds to the antigenic structure based on the domains of dengue E protein, to elicit an appropriate immune response against the quaternary structure of DENV E protein and preserve the protective capacity of EDE (Fig. 1A). The expression and purification of r2ED protein were evaluated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blotting analysis. A 27-kDa band equivalent to r2ED was visible in the purified protein sample, suggesting that the recombinant Ag had been successfully expressed (Fig. 1B). To determine whether the EDE structure is maintained in r2ED, purified r2ED was subjected to ELISA with anti-DENV EDE1 Ab (Fig. 1C). r2ED bound well to DENV EDE1-specific Ab only under nonreducing conditions without boiling or the use of reducing agents. However, neither reduced nor unreduced samples were detected by 4G2 Ab, which is capable of binding to the FLE at the extremity of domain II of DENV E protein (data not shown). The FLE is buried at the E-dimer interface; Abs targeting this FLE are cross-reactive but poorly neutralizing, and they demonstrate infection-enhancing potential. These results suggested that the r2ED protein did not display the FLE, although it retained the conformation that allowed EDE display.
Immunization with r2ED Enhances the Induction of Ag-specific and Virus-Neutralizing Ab Responses
Next, we analyzed whether r2ED was capable of inducing an efficient adaptive immune response specific to DENV EDIII Ag (Fig. 2). Examination of the EDIII-specific IgG level in serum obtained 3 days after booster immunization showed that the EDIII-specific IgG level was significantly higher in serum from mice injected with r2ED than in serum from mice injected with EDIII (1774 ± 442 vs. 330 ± 80 ng/ml, respectively, p < 0.001) (Fig. 2A). Additionally, we measured the FRNTs on DENV-susceptible Vero E6 cells using serum samples from mice immunized with PBS, EDIII, or r2ED to assess the neutralizing activity; the results showed that serum samples from mice immunized with r2ED more efficiently inhibited focus formation by DENV-2 infection, compared with serum samples from mice immunized with either PBS or EDIII (Fig. 2B). Although we confirmed that r2ED formed a dimer under nonreducing condition through SDS-PAGE followed by western blotting, there were also r2EDs which did not form dimers (data not shown). As a result, r2EDs, which did not mimic the EDE structure, were partially present, and so it is believed that the neutralization assay results were low relative to those of Ag-specific Ab production. Because high levels of viral Ag-specific Ab and robust virus-neutralizing Ab induction are associated with effective protection against viral infection (Dejnirattisai et al., 2015; Gallichotte et al., 2015), our results suggest that r2ED is capable of mediating an effective protective immune response against DENV infection.
Immunization using r2ED elicits potent Ag-specific immunity (A) and neutralizing activity against DENV-2 (B). Mice were immunized with 10 μg/mouse of the indicated Ags, and serum samples were collected 3 days after booster immunization. Representative results of triplicate experiments are shown. A Serum levels of EDIII-specific IgG determined by capture ELISA using standard mouse IgG. Results are shown as means ± SDs (n = 5). ***p < 0.001. B Serum neutralizing activity against DENV-2 infection was determined by FRNT as described in “Materials and Methods” section. After treatment with DENV-2 (3 × 103 FFU) that had been incubated with tenfold diluted serum from mice immunized with each indicated Ag, the ability to prevent DENV-2 infection in Vero E6 cells was determined by FRNT. Data are shown as means ± SDs (n = 5). *p < 0.05
r2ED Stimulates Ag-specific Activation and Cytokine Expression Involved in T-cell Immune Responses
The induction of a T-cell response is important for clinical outcomes and protection against viral infection. Additionally, T-cell memory responses encompassing broad recognition of viral proteins are important for controlling severe disease caused by viral variants. We first investigated the CD8+ T-cell response in mice after immunization by measuring the population of CD8+CD107a+ T cells after Ag stimulation, where CD107a is a functional marker for the identification of degranulated cytotoxic T lymphocytes and was present on the inner membrane of vesicles that contained perforin and granzymes (Fig. 3A). The proportion of CD8+ T cells was significantly greater in the r2ED-immunized group than in the PBS- and EDIII-immunized groups (16.3% ± 0.3% vs. 13.0% ± 0.3% vs. 9.8% ± 0.4%, respectively, all p < 0.001). Additionally, a significantly increased population of CD107a-expressing CD8+ T cells was detected in the r2ED-immunized group compared with the other experimental groups. The effects of r2ED on cellular immune response induction were further explored by assessing the pattern of cytokine expression related to initiation of the T-cell response and regulation of the Ag-specific adaptive immune response (Fig. 3B). The levels of multifunctional cytokine production, including IL-2, IL-4, and interferon-γ, were significantly higher in the EDIII-immunized group than in the PBS-immunized group. Additionally, the T-cell cytokine response after Ag treatment was significantly greater in the r2ED-immunized group than in the other groups. These results indicated that r2ED could effectively evoke both Ag-specific humoral and T cell-mediated immune responses.
Immunization using r2ED enhances Ag-specific T-cell immune response compared with immunization using EDIII. Splenocytes were collected 4 days after booster immunization with the indicated Ags, then stimulated with the indicated Ag (1 μg) for up to 20 h. Representative results of duplicate experiments are shown. A Detection of the populations of Ag-specific effector cytotoxic T lymphocytes (CTLs) by flow cytometry as described in “Materials and Methods” section. Left: representative dot plots depicting CD107a expression on spleen CD8+ T cells. Right: percentages of spleen CD8+ and CD8+CD107a+ T cells. Results are shown as means ± SDs (n = 3). ***p < 0.001. B Characterization of Ag-specific cytokine production in T cells by cytometric bead array (CBA). Amounts of cytokines in pooled cell culture supernatants (n = 3 per group) were determined as described in “Materials and Methods” section. Reactions were performed in duplicate. Data are shown as means ± SDs. *p < 0.05, **p < 0.01, ***p < 0.001
r2ED-Mediated Ag-specific Adaptive Immune Response Lasted for an Extended Period to Maintain Immune Memory
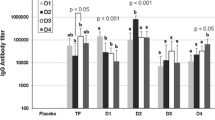
The induction of immune memory, including the persistence of Abs and protection against specific viral Ag, is essential for long-term vaccine efficacy because it ensures effective and rapid activation of a specific immune response after Ag re-exposure. To assess the ability of r2ED to promote the maintenance of Ag-specific protective immune responses, we boosted mice at 6 months after the last immunization and then measured the levels of EDIII-specific IgG and neutralizing ability of the Abs against DENV-2 infection, as well as the relative intensity and maintenance of cellular immunity (Fig. 4). The level of EDIII-specific IgG was 2.3-fold higher in the r2ED-immunized group than in the EDIII-immunized group (p < 0.05); the level in the EDIII-immunized group was significantly higher than the level in the PBS-immunized group (p < 0.01, Fig. 4A). Furthermore, serum from mice immunized with r2ED significantly reduced the FFU induced by DENV-2, compared with the FFU induced in the presence of serum from mice immunized with PBS (Fig. 4B). FFU was lower in the r2ED-immunized group than in the EDIII-immunized group, although the difference was not statistically significant. Additionally, the proportions of Ag-reactive CD4+CD44+ and CD8+CD44+ T cells were significantly greater among splenocytes from the r2ED-immunized group than among splenocytes from the PBS-immunized group (14.9% ± 0.6% vs. 8.9% ± 0.9%, respectively, p < 0.001; 15.6% ± 1.2% vs. 9.5% ± 1.7%, respectively, p < 0.01) (Fig. 4C, D). The Ag-specific CD8+CD44+ T-cell population was significantly greater in the r2ED-immunized group than in the EDIII-immunized group (p < 0.05). These results indicated that r2ED can effectively trigger the B- and T-cell responses necessary for the induction of robust neutralizing Abs and immune memory, which are critical components of vaccine efficacy.
Induction of persistent long-term Ag-specific Ab, including virus-neutralizing activity (A and B) and memory T-cell responses (C and D), after immunization using r2ED. Mice were boosted with 10 μg/mouse of the indicated Ags at 6 months after the last immunization, and serum samples were collected 3 days after booster immunization. Splenocytes were collected 4 days after booster immunization, then stimulated with Ag (1 μg) for up to 20 h. Representative results of duplicate experiments are shown. A Serum levels of EDIII-specific IgG were determined by capture ELISA using standard mouse IgG. Results are shown as means ± SDs (n = 3). *p < 0.05 and **p < 0.01. B Serum neutralizing activity against DENV-2 infection was determined by FRNT as described in the “Materials and Methods” section. After treatment with DENV-2 (3 × 103 FFU) that had been incubated with tenfold diluted sera from mice immunized with each indicated Ag, the ability to prevent DENV-2 infection in Vero E6 cells was determined by FRNT. Data are shown as means ± SDs (n = 3). *p < 0.05. Detection of the populations of Ag-specific memory CD4+CD44+ (C) and CD8+ CD44+ T cells (D) by flow cytometry as described in the “Materials and Methods” section. Left, representative dot plots depicting CD44 expression on spleen CD4+ and CD8+ T cells. Right, percentages of spleen CD4+CD44+ and CD8+CD44+ T cells. Results are shown as means ± SDs (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001
Immune Sera Induced by r2ED Showed Potent Neutralizing Activity Against Various DENV Serotypes
The EDE is a cross-reactive epitope and EDE-specific mAbs efficiently neutralize DENV-2, as well as the other serotypes of DENV. Therefore, we investigated cross-neutralizing activity against each DENV serotype using serum samples from mice immunized with r2ED (Fig. 5). We performed qRT-PCR to measure the transcript level of the C gene of each DENV serotype in Vero E6 cells after infection with virus that had been preincubated with mouse serum. We found that the serum from r2ED-immunized mice had significantly greater inhibitory activity against DENV C gene expression, compared with serum from EDIII-immunized or PBS-immunized mice. Notably, serum from r2ED-immunized mice efficiently neutralized DENV-1 to a degree comparable with the neutralization achieved using serum from EDIII-immunized mice (Fig. 5A). Furthermore, serum from r2ED-immunized mice showed superior neutralizing activity against DENV-3, compared with serum from the PBS-immunized group (p < 0.01) and the EDIII-immunized group (p < 0.05) (Fig. 5B). Additionally, serum from the r2ED-immunized group showed a significantly stronger neutralizing response against DENV-4, compared with serum from the EDIII-immunized group (p < 0.05) (Fig. 5C). Comparison of virus-neutralizing activity in Vero E6 cells by FRNT also showed that the sera from r2ED-immunized mice exhibited efficient neutralizing potency in DENV-3 and -4 infection (data not shown). Notably, serum from r2ED-immunized mice showed significantly greater neutralizing activity against DENV-4, compared with serum from other groups; there was no difference between the PBS-immunized and EDIII-immunized groups. Taken together, these results indicate that r2ED, which mimics the quaternary structure of DENV E protein dimer, elicits cross-reactive neutralizing Abs against all DENV serotypes that are superior to the Abs elicited by EDIII. Moreover, r2ED is capable of promoting a protective immune response against DENV infection.
Immunization using r2ED provides cross-reactive immune responses toward DENV-1/3/4. DENV C gene transcript levels in Vero E6 cells at 24 h after treatment with DENV-1, -3, and -4 (3 × 103 FFU) that had been incubated with 100-fold diluted sera from mice immunized with each indicated Ag. qRT-PCR was performed as described in the “Materials and Methods” section. Values were normalized relative to the expression of the internal control gene (Vero E6 β-actin). We set the sera-untreated virus alone as the reference, and the value for the sample as 1. Reactions were performed in triplicate. Results are shown as means ± SDs (n = 3). *p < 0.05, **p < 0.01
Discussion
There have been multiple attempts to develop effective DENV vaccines capable of inducing safe, long-lasting, and cross-reactive immune responses to all four serotypes of DENV (Araujo et al., 2020). The differences between vaccine and circulating DENV strains may be responsible for the low efficacies of vaccines against specific DENV serotypes, as demonstrated using Dengvaxia® and TAK-003 (Biswal et al., 2020; Hadinegoro et al., 2015). Barriers to DENV vaccine development may be resolved by using the quaternary structure of Ags to support the induction of efficient neutralizing, cross-protective, and elevated Ab responses (Murphy & Whitehead, 2011; Park et al., 2022). Here, we demonstrated the feasibility of using r2ED to induce neutralizing Abs directed against EDE that bridges two envelope proteins of DENV. This strategy has the potential to generate a protein subunit vaccine capable of inducing cross-reactive Abs and possibly protecting against infection by multiple DENV serotypes.
Humoral immune responses to DENV are dominated by cross-reactive but weakly neutralizing Abs against conserved FLE; accordingly, the antigenic heterogeneity of infected DENV appears to increase the possibility of inducing ADE (Cherrier et al., 2009; Dejnirattisai et al., 2010; Lai et al., 2008). EDE is a quaternary epitope formed by EDII and EDIII on two different monomers within a single dimer (Fibriansah et al., 2015). Abs for EDE are reportedly the most potent and broadly reactive neutralizing Abs against DENVs. Consequently, the immune response elicited by the EDE region is considered ideal to achieve simultaneous protection against four DENV serotypes in vaccine design, because EDEs can be formed by the E-dimer interface and overlay the FLE, thus limiting the FLE exposure to avoid the poor neutralizing and infection-enhancing characteristics of FLE (Park et al., 2020). However, the EDE region contains complex epitopes extensively distributed within E protein and conformationally expressed in E dimers. Here, we constructed an r2ED Ag to generate an Ag of reduced size compared with the full-length E protein and expressing the major epitopes recognized by anti-EDE Abs, which exhibited reduced binding capacity when amino acids in DENV-2 E-dimer structure were substituted (Dejnirattisai et al., 2015). We demonstrated that r2ED, a recombinant Ag designed based on DENV-2 E protein, did not alter the conformational epitope of the E dimer (Fig. 1) was not recognized by anti-FLE mAb (data not shown). These results suggested that FLE is not displayed on the surface of r2ED, thus excluding the possibility of eliciting Abs specific to FLE that are capable of inducing ADE. Furthermore, we found that the sera from r2ED-immunized mice did not induce ADE in IgG Fc receptor-expressing cells, in contrast to the sera from EDIII-immunized mice and FLE-specific mAb 4G2 (Fig. S1). Importantly, 4G2 can cross-react with all four DENV serotypes, but its binding exhibits low avidity and promotes the entry of all four DENV serotypes (Beltramello et al., 2010; Smith et al., 2012). Unlike the FLE, which elicits weakly or non-neutralizing Abs, the EDE generally elicits virus-neutralizing Abs and exhibits cross-reactivity. We also showed that r2ED-immunized serum significantly neutralized DENV-2, as well as other DENV serotypes (Fig. 5). Although less effective than r2ED-immunized serum, EDIII-immunized serum also tended to reduce the infectivities of DENV-1 and DENV-3, but not DENV-4, presumably because of differences in DIII domain amino acid sequences among the four DENV serotypes. This speculation was supported by the results of phylogenetic studies conducted on numerous DENV epidemic and sylvatic strains, which revealed that DENV-1 and DENV-3 share amino acid sequence similarity of approximately 60–70% and are closer to DENV-2 than to DENV-4. In contrast, DENV-4 has only 40–50% amino acid sequence similarity with other DENV serotypes (Wong et al., 2018). We confirmed that the quaternary structure epitope-containing r2ED is effective in maintaining long-lasting Ag-specific durable Ab responses and T-cell responses (Fig. 4). Another study showed that quaternary epitopes on DENV E protein homodimers are the major targets of long-lasting polyclonal neutralizing Abs generated by DENV infection (Gallichotte et al., 2015).
We next examined whether r2ED was capable of inducing protective immune responses against DENV-2 infection in AG129 mice (interferon α/β/γ R−/−), which are susceptible to infection by various flaviviruses, including DENV (Baldon et al., 2022). Although we found no changes in survival after challenge infection with DENV-2 among AG129 mice immunized with different Ags, presumably because submaximal viral titers were used to monitor disease severity, we showed that EDIII-specific IgG level and neutralizing activity against DENV-2 were significantly higher in the serum of AG129 mice immunized with r2ED than in the serum of AG129 mice immunized with EDIII alone and in the serum of control AG129 mice (p < 0.001) (Fig. S2A, B). Additionally, disseminated DENV-2 infection was reduced in r2ED-immunized AG129 mice, such that the levels of viral RNA in the spleens of these mice were significantly lower than in the other groups, as indicated by the decline of DENV C gene expression at 3 days post-infection (Fig. S2C). These results suggested that r2ED is capable of inducing a potent viral Ag-specific immune response with protective activity against DENV infection; the immune response can also prevent disease development and viral dissemination after infection. Taken together, our results suggest that r2ED can elicit a cross-reactive and durable neutralizing Ab response against DENV without enhancing virus infection, although further studies are needed to determine whether r2ED can effectively prevent infection by DENV-2 and the other three DENV serotypes in AG129 mice.
High levels of viral Ag-specific adaptive immune responses and enhanced virus neutralization ability are both associated with the protective efficacies of vaccines against viral infection. Furthermore, a recombinant vaccine engineered to mimic conformational epitopes and minimize FLE display is more likely to induce a balanced immune response against the four DENV serotypes with reduced incidence of ADE. In this study, we demonstrated the capacity to produce r2ED, which displays the EDE and is not recognized by mAbs targeting the FLE. r2ED was based on the DENV E structure consisting of three different domains; it was capable of inducing virus-specific protective immune responses, including long-term humoral and cellular immune responses. r2ED, which expresses the quaternary neutralizing epitope present in all four DENV serotypes, may be useful in the development of a protein-based vaccine that is simpler and more effective than currently available tetravalent dengue vaccine formulations.
Data availability
All data generated or analysed during this study are available in the Dryad repository, https://doi.org/10.1007/s12275-023-00021-z.
References
Araujo, S. C., Pereira, L. R., Alves, R. P. S., Andreata-Santos, R., Kanno, A. I., Ferreira, L. C. S., & Gonçalves, V. M. (2020). Anti-flavivirus vaccines: Review of the present situation and perspectives of subunit vaccines produced in Escherichia coli. Vaccines, 8, 492.
Baldon, L. V. R., de Mendonça, S. F., Ferreira, F. V., Rezende, F. O., Amadou, S. C. G., Leite, T. H. J. F., Rocha, M. N., Marques, J. T., Moreira, L. A., & Ferreira, A. G. A. (2022). AG129 mice as a comprehensive model for the experimental assessment of mosquito vector competence for arboviruses. Pathogens, 11, 879.
Balmaseda, A., Hammond, S. N., Perez, L., Tellez, Y., Saborio, S. I., Mercado, J. C., Cuadra, R., Rocha, J., Perez, M. A., Silva, S., et al. (2006). Serotype-specific differences in clinical manifestations of dengue. American Journal of Tropical Medicine and Hygiene, 74, 449–456.
Barba-Spaeth, G., Dejnirattisai, W., Rouvinski, A., Vaney, M. C., Medits, I., Sharma, A., Simon-Lorière, E., Sakuntabhai, A., Cao-Lormeau, V. M., Haouz, A., et al. (2016). Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature, 536, 48–53.
Beltramello, M., Williams, K. L., Simmons, C. P., MacAgno, A., Simonelli, L., Quyen, N. T. H., Sukupolvi-Petty, S., Navarro-Sanchez, E., Young, P. R., de Silva, A. M., et al. (2010). The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host & Microbe, 8, 271–283.
Bhatt, S., Gething, P. W., Brady, O. J., Messina, J. P., Farlow, A. W., Moyes, C. L., Drake, J. M., Brownstein, J. S., Hoen, A. G., Sankoh, O., et al. (2013). The global distribution and burden of dengue. Nature, 496, 504–507.
Biswal, S., Borja-Tabora, C., Martinez Vargas, L., Velásquez, H., Theresa Alera, M., Sierra, V., Rodriguez-Arenales, E. J., Yu, D., Wickramasinghe, V. P., Moreira, E. D., Jr., et al. (2020). Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: A randomised, placebo-controlled, phase 3 trial. Lancet, 395, 1423–1433.
Cherrier, M. V., Kaufmann, B., Nybakken, G. E., Lok, S. M., Warren, J. T., Chen, B. R., Nelson, C. A., Kostyuchenko, V. A., Holdaway, H. A., Chipman, P. R., et al. (2009). Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO Journal, 28, 3269–3276.
Dejnirattisai, W., Jumnainsong, A., Onsirisakul, N., Fitton, P., Vasanawathana, S., Limpitikul, W., Puttikhunt, C., Edwards, C., Duangchinda, T., Supasa, S., et al. (2010). Cross-reacting antibodies enhance dengue virus infection in humans. Science, 328, 745–748.
Dejnirattisai, W., Wongwiwat, W., Supasa, S., Zhang, X., Dai, X., Rouvinski, A., Jumnainsong, A., Edwards, C., Quyen, N. T. H., Duangchinda, T., et al. (2015). A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nature Immunology, 16, 170–177.
Fernandez, E., Dejnirattisai, W., Cao, B., Scheaffer, S. M., Supasa, P., Wongwiwat, W., Esakky, P., Drury, A., Mongkolsapaya, J., Moley, K. H., et al. (2017). Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nature Immunology, 18, 1261–1269.
Fibriansah, G., Ibarra, K. D., Ng, T. S., Smith, S. A., Tan, J. L., Lim, X. N., Ooi, J. S., Kostyuchenko, V. A., Wang, J., de Silva, A. M., et al. (2015). Dengue virus. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science, 349, 88–91.
Fried, J. R., Gibbons, R. V., Kalayanarooj, S., Thomas, S. J., Srikiatkhachorn, A., Yoon, I. K., Jarman, R. G., Green, S., Rothman, A. L., & Cummings, D. A. (2010). Serotype-specific differences in the risk of dengue hemorrhagic fever: An analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Neglected Tropical Diseases, 4, e617.
Gallichotte, E. N., Widman, D. G., Yount, B. L., Wahala, W. M., Durbin, A., Whitehead, S., Sariol, C. A., Crowe, J. E., Jr., de Silva, A. M., & Baric, R. S. (2015). A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. Mbio, 6, e01461-e1515.
Hadinegoro, S. R., Arredondo-García, J. L., Capeding, M. R., Deseda, C., Chotpitayasunondh, T., Dietze, R., Hj Muhammad Ismail, H. I., Reynales, H., Limkittikul, K., Rivera-Medina, D. M., et al. (2015). Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. New England Journal of Medicine, 373, 1195–1206.
Halstead, S. B. (2017). Personal view achieving safe, effective, and durable Zika virus vaccines: Lessons from dengue. The Lancet Infectious Diseases, 17, E378–E382.
Lai, C. Y., Tsai, W. Y., Lin, S. R., Kao, C. L., Hu, H. P., King, C. C., Wu, H. C., Chang, G. J., & Wang, W. K. (2008). Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. Journal of Virology, 82, 6631–6643.
Li, X. Q., Qiu, L. W., Chen, Y., Wen, K., Cai, J. P., Chen, J., Pan, Y. X., Li, J., Hu, D. M., Huang, Y. F., et al. (2013). Dengue virus envelope domain III immunization elicits predominantly cross-reactive, poorly neutralizing antibodies localized to the AB loop: Implications for dengue vaccine design. Journal of General Virology, 94, 2191–2201.
Messina, J. P., Brady, O. J., Golding, N., Kraemer, M. U. G., Wint, G. R. W., Ray, S. E., Pigott, D. M., Shearer, F. M., Johnson, K., Earl, L., et al. (2019). The current and future global distribution and population at risk of dengue. Nature Microbiology, 4, 508–1515.
Midgley, C. M., Flanagan, A., Tran, H. B., Dejnirattisai, W., Chawansuntati, K., Jumnainsong, A., Wongwiwat, W., Duangchinda, T., Mongkolsapaya, J., Grimes, J. M., et al. (2012). Structural analysis of a dengue cross-reactive antibody complexed with envelope domain III reveals the molecular basis of cross-reactivity. The Journal of Immunology, 188, 4971–4979.
Ministry of Health. (2012). Communicable Disease Surveillance in Singapore 2011. Ministry of Health, Singapore.
Murphy, B. R., & Whitehead, S. S. (2011). Immune response to dengue virus and prospects for a vaccine. Annual Review of Immunology, 29, 587–619.
Park, J., Kim, J., & Jang, Y. S. (2022). Current status and perspectives on vaccine development against dengue virus infection. Journal of Microbiology, 60, 247–254.
Park, J., Lee, H. Y., Khai, L. T., Thuy, N. T. T., Mai, L. Q., & Jang, Y. S. (2020). Addition of partial envelope domain II into envelope domain III of dengue virus antigen potentiates the induction of virus-neutralizing antibodies and induces protective immunity. Vaccines, 8, 88.
Paudel, D., Jarman, R., Limkittikul, K., Klungthong, C., Chamnanchanunt, S., Nisalak, A., Gibbons, R., & Chokejindachai, W. (2011). Comparison of real-time SYBR green dengue assay with real-time taqman RT-PCR dengue assay and the conventional nested PCR for diagnosis of primary and secondary dengue infection. North American Journal of Medical Sciences, 3, 478–485.
Pinheiro-Michelsen, J. R., Souza, R. D. S. O., Santana, I. V. R., da Silva, P. S., Mendez, E. C., Luiz, W. B., & Amorim, J. H. (2020). Anti-dengue vaccines: From development to clinical trials. Frontiers in Immunology, 11, 1252.
Priyamvada, L., Cho, A., Onlamoon, N., Zheng, N. Y., Huang, M., Kovalenkov, Y., Chokephaibulkit, K., Angkasekwinai, N., Pattanapanyasat, K., Ahmed, R., et al. (2016). B cell responses during secondary dengue virus infection are dominated by highly cross-reactive, memory-derived plasmablasts. Journal of Virology, 90, 5574–5585.
Rothman, A. L. (2011). Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nature Reviews Immunology, 11, 532–543.
Rouvinski, A., Dejnirattisai, W., Guardado-Calvo, P., Vaney, M. C., Sharma, A., Duquerroy, S., Supasa, P., Wongwiwat, W., Haouz, A., Barba-Spaeth, G., et al. (2017). Covalently linked dengue virus envelope glycoprotein dimers reduce exposure of the immunodominant fusion loop epitope. Nature Communications, 8, 15411.
Shukla, R., Ramasamy, V., Shanmugam, R. K., Ahuja, R., & Khanna, N. (2020). Antibody-dependent enhancement: A challenge for developing a safe dengue vaccine. Frontiers in Cellular and Infection Microbiology, 10, 572681.
Smith, S. A., Zhou, Y., Olivarez, N. P., Broadwater, A. H., de Silva, A. M., & Crowe, J. E. (2012). Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. Journal of Virology, 86, 2665–2675.
Swaminathan, S., & Khanna, N. (2019). Dengue vaccine development: Global and Indian scenarios. International Journal of Infectious Diseases, 84S, S80–S86.
Thomas, A., Thiono, D. J., Kudlacek, S. T., Forsberg, J., Premkumar, L., Tian, S., Kuhlman, B., de Silva, A. M., & Metz, S. W. (2020). Dimerization of dengue virus E subunits impacts antibody function and domain focus. Journal of Virology, 94, e00745-e820.
Wong, Y. H., Kumar, A., Liew, C. W., Tharakaraman, K., Srinivasaraghavan, K., Sasisekharan, R., Verma, C., & Lescar, J. (2018). Molecular basis for dengue virus broad cross-neutralization by humanized monoclonal antibody 513. Science and Reports, 8, 8449.
Yung, C. F., Lee, K. S., Thein, T. L., Tan, L. K., Gan, V. C., Wong, J. G. X., Lye, D. C., Ng, L. C., & Leo, Y. S. (2015). Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. The American Journal of Tropical Medicine and Hygiene, 92, 999–1005.
Acknowledgements
This work was supported by the Basic Science Research Programs (2017R1A6A1A03015876 to Y.-S. Jang and 2019R1I1A3A01062224 to J. Kim) through the National Research Foundation (NRF) of Korea funded by the Ministry of Education. Dr. J. Park and Ms. T. Y. Lim were supported by the BK21 FOUR program in the Department of Bioactive Material Sciences. Some experiments were performed using the instruments installed in the Center for University-Wide Research Facilities (CURF) at Jeonbuk National University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest to declare.
Ethical statement
Animal experiments were approved and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Jeonbuk National University (Approval No. CBNU 2020–018).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J., Lim, T.Y., Park, J. et al. Recombinant Protein Mimicking the Antigenic Structure of the Viral Surface Envelope Protein Reinforces Induction of an Antigen-Specific and Virus-Neutralizing Immune Response Against Dengue Virus. J Microbiol. 61, 131–143 (2023). https://doi.org/10.1007/s12275-023-00021-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-023-00021-z