Abstract
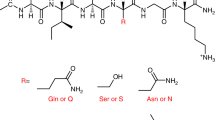
Although the relationship between molecular and supramolecular chirality remains elusive, the existing results have demonstrated the vital role of hydrophilic motifs in controlling the supramolecular handedness of peptide nanofibrils compared with hydrophobic ones. However, unlike conventional hydrophobic residues, we speculate that aromatic hydrophobic residues are mostly likely to play a unique role in regulating the supramolecular handedness because the π−π stacking interactions of their side chains are directional like hydrogen bonding and can direct high levels of self-assembly due to the geometric confining of aromatic rings. To confirm this hypothesis, we here design a series of amphiphilic short peptides, with their hydrophobic motifs being composed of aromatic residues. Their short lengths not only favor their structural stability, synthesis, and sequence variation but also enable us to readily link their molecular and supramolecular structures. Through the combination of experiments and theoretical simulations, we demonstrate that the peptides containing L-form aromatic residues form left-handed nanofibrils while those containing D-form aromatic residues assemble into right-handed ones, irrespective of the chirality of their C-terminal hydrophilic residue. Theoretical calculations revealed that the stacking of aromatic side chains between β-strands directed the twisting direction of the β-sheets formed, with L- and D-form phenylalanine side chains stacking in a clockwise and anti-clockwise way, and more ordered and stronger aromatic stacking for homochiral peptides facilitated the formation of nanofibrils with a marked tubular feature. This study has bridged the knowledge gap in our understanding of how aromatic residues affect the supramolecular chirality of short peptides.

Similar content being viewed by others
References
Feringa, B. L.; van Delden, R. A. Absolute asymmetric synthesis: The origin, control, and amplification of chirality. Angew. Chem., Int. Ed. 1999, 38, 3418–3438.
Liu, G. F.; Zhang, D.; Feng, C. L. Control of three-dimensional cell adhesion by the chirality of nanofibers in hydrogels. Angew. Chem., Int. Ed. 2014, 53, 7789–7793.
Harper, J. D.; Lieber, C. M.; Lansbury, P. T. Jr. Atomic force microscopic imaging of seeded fibril formation and fibril branching by the Alzheimer’s disease amyloid-β protein. Chem. Biol. 1997, 4, 951–959.
Goldsbury, C. S.; Cooper, G. J. S.; Goldie, K. N.; Müller, S. A.; Saafi, E. L.; Gruijters, W. T. M.; Misur, M. P.; Engel, A.; Aebi, U.; Kistler, J. Polymorphic fibrillar assembly of human amylin. J. Struct. Biol. 1997, 119, 17–27.
Jiménez, J. L.; Nettleton, E. J.; Bouchard, M.; Robinson, C. V.; Dobson, C. M.; Saibil, H. R. The protofilament structure of insulin amyloid fibrils. Proc. Natl. Acad. Sci. USA 2002, 99, 9196–9201.
Bedrood, S.; Li, Y. Y.; Isas, J. M.; Hegde, B. G.; Baxa, U.; Haworth, I. S.; Langen, R. Fibril structure of human islet amyloid polypeptide. J. Biol. Chem. 2012, 287, 5235–5241.
Schmidt, A.; Annamalai, K.; Schmidt, M.; Grigorieff, N.; Fändrich, M. Cryo-EM reveals the steric zipper structure of a light chain-derived amyloid fibril. Proc. Natl. Acad. Sci. USA 2016, 113, 6200–6205.
Close, W.; Neumann, M.; Schmidt, A.; Hora, M.; Annamalai, K.; Schmidt, M.; Reif, B.; Schmidt, V.; Grigorieff, N.; Fändrich, M. Physical basis of amyloid fibril polymorphism. Nat. Commun. 2018, 9, 699.
Rubin, N.; Perugia, E.; Goldschmidt, M.; Fridkin, M.; Addadi, L. Chirality of amyloid suprastructures. J. Am. Chem. Soc. 2008, 130, 4602–4603.
Kurouski, D.; Dukor, R. K.; Lu, X. F.; Nafie, L. A.; Lednev, I. K. Normal and reversed supramolecular chirality of insulin fibrils probed by vibrational circular dichroism at the protofilament level of fibril structure. Biophys. J. 2012, 103, 522–531.
Kurouski, D.; Lu, X. F.; Popova, L.; Wan, W.; Shanmugasundaram, M.; Stubbs, G.; Dukor, R. K.; Lednev, I. K.; Nafie, L. A. Is supramolecular filament chirality the underlying cause of major morphology differences in amyloid fibrils. J. Am. Chem. Soc. 2014, 136, 2302–2312.
Usov, I.; Adamcik, J.; Mezzenga, R. Polymorphism complexity and handedness inversion in serum albumin amyloid fibrils. ACS Nano 2013, 7, 10465–10474.
Rubin, N.; Perugia, E.; Wolf, S. G.; Klein, E.; Fridkin, M.; Addadi, L. Relation between serum amyloid A truncated peptides and their suprastructure chirality. J. Am. Chem. Soc. 2010, 132, 4242–4248.
Lara, C.; Reynolds, N. P.; Berryman, J. T.; Xu, A. Q.; Zhang, A. F.; Mezzenga, R. ILQINS hexapeptide, identified in lysozyme left-handed helical ribbons and nanotubes, forms right-handed helical ribbons and crystals. J. Am. Chem. Soc. 2014, 136, 4732–4739.
Reynolds, N. P.; Adamcik, J.; Berryman, J. T.; Handschin, S.; Zanjani, A. A. H.; Li, W.; Liu, K.; Zhang, A. F.; Mezzenga, R. Competition between crystal and fibril formation in molecular mutations of amyloidogenic peptides. Nat. Commun. 2017, 8, 1338.
Wang, M.; Zhou, P.; Wang, J. Q.; Zhao, Y. R.; Ma, H. C.; Lu, J. R.; Xu, H. Left or right: How does amino acid chirality affect the handedness of nanostructures self-assembled from short amphiphilic peptides. J. Am. Chem. Soc. 2017, 139, 4185–4194.
Fu, Y. T.; Li, B. Z.; Huang, Z. B.; Li, Y.; Yang, Y. G. Terminal is important for the helicity of the self-assemblies of dipeptides derived from alanine. Langmuir 2013, 29, 6013–6017.
Gazit, E. A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002, 16, 77–83.
Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627.
Mehta, A. K.; Lu, K.; Childers, W. S.; Liang, Y.; Dublin, S. N.; Dong, J. J.; Snyder, J. P.; Pingali, S. V.; Thiyagarajan, P.; Lynn, D. G. Facial symmetry in protein self-assembly. J. Am. Chem. Soc. 2008, 130, 9829–9835.
Smith, A. M.; Williams, R. J.; Tang, C.; Coppo, P.; Collins, R. F.; Turner, M. L.; Saiani, A.; Ulijn, R. V. Fmoc-diphenylalanine self assembles to a hydrogel via a novel architecture based on π−π interlocked β-sheets. Adv. Mater. 2008, 20, 37–41.
Li, J.; Wang, J. Q.; Zhao, Y. R.; Zhou, P.; Carter, J.; Li, Z. Y.; Waigh, T. A.; Lu, J. R.; Xu, H. Surfactant-like peptides: From molecular design to controllable self-assembly with applications. Coord. Chem. Rev. 2020, 421, 213418.
Wang, M. H.; Zhao, Y. R.; Zhang, L. M.; Deng, J.; Qi, K.; Zhou, P.; Ma, X. Y.; Wang, D.; Li, Z.; Wang, J. Q. et al. Unexpected role of achiral glycine in determining the suprastructural handedness of peptide nanofibrils. ACS Nano 2021, 15, 10328–10341.
Wang, F. B.; Gnewou, O.; Solemanifar, A.; Conticello, V. P.; Egelman, E. H. Cryo-EM of helical polymers. Chem. Rev. 2022, 122, 14055–14065.
Usov, I.; Mezzenga, R. FiberApp: An open-source software for tracking and analyzing polymers, filaments, biomacromolecules, and fibrous objects. Macromolecules 2015, 48, 1269–1280.
Levine, H. Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410.
Biancalana, M.; Makabe, K.; Koide, A.; Koide, S. Molecular mechanism of thioflavin-T binding to the surface of β-rich peptide self-assemblies. J. Mol. Biol. 2009, 385, 1052–1063.
Pelton, J. T.; McLean, L. R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000, 277, 167–176.
Marchesan, S.; Easton, C. D.; Kushkaki, F.; Waddington, L.; Hartley, P. G. Tripeptide self-assembled hydrogels: Unexpected twists of chirality. Chem. Commun. 2012, 48, 2195–2197.
Marchesan, S.; Easton, C. D.; Styan, K. E.; Waddington, L. J.; Kushkaki, F.; Goodall, L.; McLean, K. M.; Forsythe, J. S.; Hartley, P. G. Chirality effects at each amino acid position on tripeptide self-assembly into hydrogel biomaterials. Nanoscale 2014, 6, 5172–5180.
Lee, N. R.; Bowerman, C. J.; Nilsson, B. L. Effects of varied sequence pattern on the self-assembly of amphipathic peptides. Biomacromolecules 2013, 14, 3267–3277.
Lin, S. W.; Li, Y.; Li, B. Z.; Yang, Y. G. Control of the handedness of self-assemblies of dipeptides by the chirality of phenylalanine and steric hindrance of phenylglycine. Langmuir 2016, 32, 7420–7426.
Behanna, H. A.; Donners, J. J. J. M.; Gordon, A. C.; Stupp, S. I. Coassembly of amphiphiles with opposite peptide polarities into nanofibers. J. Am. Chem. Soc. 2005, 127, 1193–1200.
Knaapila, M.; Almásy, L.; Garamus, V. M.; Ramos, M. L.; Justino, L. L. G.; Galbrecht, F.; Preis, E.; Scherf, U.; Burrows, H. D.; Monkman, A. P. An effect of side chain length on the solution structure of poly(9, 9-dialkylfluorene)s in toluene. Polymer 2008, 49, 2033–2038.
Zhao, Y. R.; Hu, X. Z.; Zhang, L. M.; Wang, D.; King, S. M.; Rogers, S. E.; Wang, J. Q.; Lu, J. R.; Xu, H. Monolayer wall nanotubes self-assembled from short peptide bolaamphiphiles. J. Colloid Interface Sci. 2021, 583, 553–562.
Ma, X. Y.; Zhao, Y. R.; He, C. Y.; Zhou, X.; Qi, H.; Wang, Y.; Chen, C. X.; Wang, D.; Li, J.; Ke, Y. B. et al. Ordered packing of β-sheet nanofibrils into nanotubes: Multi-hierarchical assembly of designed short peptides. Nano Lett. 2021, 21, 10199–10207.
Lu, K.; Jacob, J.; Thiyagarajan, P.; Conticello, V. P.; Lynn, D. G. Exploiting amyloid fibril lamination for nanotube self-assembly. J. Am. Chem. Soc. 2003, 125, 6391–6393.
Zhao, Y. R.; Yang, W.; Wang, D.; Wang, J. Q.; Li, Z. Y.; Hu, X. Z.; King, S.; Rogers, S.; Lu, J. R.; Xu, H. Controlling the diameters of nanotubes self-assembled from designed peptide bolaphiles. Small 2018, 14, 1703216.
da Silva, E. R.; Alves, W. A.; Castelletto, V.; Reza, M.; Ruokolainen, J.; Hussain, R.; Hamley, I. W. Self-assembly pathway of peptide nanotubes formed by a glutamatic acid-based bolaamphiphile. Chem. Commun. 2015, 51, 11634–11637.
Sunde, M.; Blake, C. C. F. From the globular to the fibrous state: Protein structure and structural conversion in amyloid formation. Q. Rev. Biophys. 1998, 31, 1–39.
Pashuck, E. T.; Stupp, S. I. Direct observation of morphological tranformation from twisted ribbons into helical ribbons. J. Am. Chem. Soc. 2010, 132, 8819–8821.
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19.
Jorgensen, W. L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666.
Jorgensen, W. L.; Maxwell, D. S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236.
Berendsen, H. J. C.; Grigera, J. R.; Straatsma, T. P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271.
Mayans, E.; Casanovas, J.; Gil, A. M.; Jiménez, A. I.; Cativiela, C.; Puiggalí, J.; Alemán, C. Diversity and hierarchy in supramolecular assemblies of triphenylalanine: From laminated helical ribbons to toroids. Langmuir 2017, 33, 4036–4048.
Lu, T.; Chen, Q. X. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555.
Lu, T.; Chen, F. W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592.
Acknowledgements
This work was supported by the National Natural Science Foundation (Nos. 22172193, 22072181, and U1832108), a joint Innovate UK-Syngenta funded project under knowledge transfer partnership (No. KTP12697) and an EPSRC IAA 377 grant (No. R128362) with Arxada. We acknowledge the use of the resources of the China Spallation Neutron Source in Dongguan of Guangdong Province of China.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Qi, H., Qi, K., Li, J. et al. The role of aromatic residues in controlling the supramolecular chirality of short amphiphilic peptides. Nano Res. 16, 12230–12237 (2023). https://doi.org/10.1007/s12274-023-5783-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5783-y