Abstract
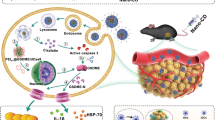
Despite recent advances in melanoma treatment through the use of antibody immunotherapy, the clinical benefit remains restricted by its inefficient infiltration and immunosuppression within the tumor microenvironment (TME). In addition, immune-related adverse events (irAEs) have often occurred due to the off-target binding of therapeutic drugs to normal tissues after systematic administration. Herein, we constructed an integrated and cascaded drug delivery system for the treatment of melanoma. In addition to blocking the programmed cell death protein 1 or its ligand (PD-1/PD-L1) axis, the PD-L1 targeting peptide (FE) with spherical micelle self-assembly characteristics could also effectively encapsulate the immune adjuvant resiquimod (R848), and form a complete nano drug. FER was further integrated into tumor-responsive microneedles (MNs) to establish FER@MN and could reach the cascaded functions. FER could be sustainedly released from the MN system and disassemble into monomers, achieving PD-1/PD-L1 axis blockade whilst reprogramming the immunosuppressive TME. Notably, FER@MN permits the controllable release and retention enhancement of the targeting peptide in the TME, thus causing prolonged PD-L1 blockade effect. It is demonstrated that this synergistic treatment could efficiently inhibit melanoma growth, providing a new strategy for the combination treatment of melanoma.

Similar content being viewed by others
References
Häuser, W.; Ablin, J.; Fitzcharles, M. A.; Littlejohn, G.; Luciano, J. V.; Usui, C.; Walitt, B. Fibromyalgia. Nat. Rev. Dis. Primers 2015, 1, 15022.
Bu, J.; Nair, A.; Iida, M.; Jeong, W. J.; Poellmann, M. J.; Mudd, K.; Kubiatowicz, L. J.; Liu, E. W.; Wheeler, D. L.; Hong, S. An avidity-based PD-L1 antagonist using nanoparticle-antibody conjugates for enhanced immunotherapy. Nano Lett. 2020, 20, 4901–4909.
Zhang, X. D.; Wang, J. Q.; Chen, Z. W.; Hu, Q. Y.; Wang, C.; Yan, J. J.; Dotti, G.; Huang, P.; Gu, Z. Engineering PD-1-presenting platelets for cancer immunotherapy. Nano Lett. 2018, 18, 5716–5725.
Nam, J.; Son, S.; Park, K. S.; Zou, W. P.; Shea, L. D.; Moon, J. J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414.
Topalian, S. L.; Taube, J. M.; Anders, R. A.; Pardoll, D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287.
Sharma, P.; Hu-Lieskovan, S.; Wargo, J. A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723.
Hu, M.; Zhang, J.; Yu, Y. L.; Tu, K.; Yang, T.; Wang, Y.; Hu, Q.; Kong, L.; Zhang, Z. P. Injectable liquid crystal formation system for reshaping tumor immunosuppressive microenvironment to boost antitumor immunity: Postoperative chemoimmunotherapy. Small 2020, 16, 2004905.
Sun, M. Q.; Yao, S. B.; Fan, L. Y.; Fang, Z. G.; Miao, W. B.; Hu, Z. Y.; Wang, Z. H. Fibroblast activation protein-α responsive peptide assembling prodrug nanoparticles for remodeling the immunosuppressive microenvironment and boosting cancer immunotherapy. Small 2022, 18, 2106296.
Jeong, W. J.; Choi, S. H.; Jin, K. S.; Lim, Y. B. Tuning oligovalent biomacromolecular interfaces using double-layered α-helical coiled-coil nanoassemblies from lariat-type building blocks. ACS Macro Lett. 2016, 5, 1406–1410.
Lau, J. L.; Dunn, M. K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707.
Yin, H. W.; Zhou, X. M.; Huang, Y. H.; King, G. J.; Collins, B. M.; Gao, Y. F.; Craik, D. J.; Wang, C. K. Rational design of potent peptide inhibitors of the PD-1: PD-L1 interaction for cancer immunotherapy. J. Am. Chem. Soc. 2021, 143, 18536–18547.
Fu, L. P.; Zhang, J. H.; Wu, C. C.; Wang, W. Z.; Wang, D.; Hu, Z. Y.; Wang, Z. H. A novel PD-L1 targeting peptide self-assembled nanofibers for sensitive tumor imaging and photothermal immunotherapy in vivo. Nano Res. 2022, 15, 7286–7294.
Zhang, C.; Gao, F.; Wu, W.; Qiu, W. X.; Zhang, L.; Li, R. Q.; Zhuang, Z. N.; Yu, W. Y.; Cheng, H.; Zhang, X. Z. Enzyme-driven membrane-targeted chimeric peptide for enhanced tumor photodynamic immunotherapy. ACS Nano 2019, 13, 11249–11262.
Xiao, W. Y.; Wang, Y.; An, H. W.; Hou, D. Y.; Mamuti, M.; Wang, M. D.; Wang, J.; Xu, W. H.; Hu, L. M.; Wang, H. Click reaction-assisted peptide immune checkpoint blockade for solid tumor treatment. ACS Appl. Mater. Interfaces 2020, 12, 40042–40051.
Zhang, L. G.; Su, T.; He, B.; Gu, Z. W. Self-assembly polyrotaxanes nanoparticles as carriers for anticancer drug methotrexate delivery. Nano-Micro Lett. 2014, 6, 108–115.
Xia, X. L.; Yang, X. Y.; Huang, W.; Xia, X. X.; Yan, D. Y. Self-assembled nanomicelles of affibody-drug conjugate with excellent therapeutic property to cure ovary and breast cancers. Nano-Micro Lett. 2022, 14, 33.
Dong, R. J.; Zhou, Y. F.; Huang, X. H.; Zhu, X. Y.; Lu, Y. F.; Shen, J. Functional supramolecular polymers for biomedical applications. Adv. Mater. 2015, 27, 498–526.
Cheng, H.; Cheng, Y. J.; Bhasin, S.; Zhu, J. Y.; Xu, X. D.; Zhuo, R. X.; Zhang, X. Z. Complementary hydrogen bonding interaction triggered co-assembly of an amphiphilic peptide and an anti-tumor drug. Chem. Commun. 2015, 51, 6936–6939.
Zou, R. F.; Wang, Q.; Wu, J. C.; Wu, J. X.; Schmuck, C.; Tian, H. Peptide self-assembly triggered by metal ions. Chem. Soc. Rev. 2015, 44, 5200–5219.
Xu, L.; Shen, Q.; Huang, L. Z.; Xu, X. D.; He, H. Y. Chargemediated co-assembly of amphiphilic peptide and antibiotics into supramolecular hydrogel with antibacterial activity. Front. Bioeng. Biotechnol. 2020, 8, 629452.
Cao, M. W.; Lu, S.; Wang, N. N.; Xu, H.; Cox, H.; Li, R. H.; Waigh, T.; Han, Y. C.; Wang, Y. L.; Lu, J. R. Enzyme-triggered morphological transition of peptide nanostructures for tumor-targeted drug delivery and enhanced cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 16357–16366.
Ren, C. H.; Gao, Y.; Guan, Y.; Wang, Z. Y.; Yang, L. J.; Gao, J.; Fan, H. R.; Liu, J. F. Carrier-free supramolecular hydrogel composed of dual drugs for conquering drug resistance. ACS Appl. Mater. Interfaces 2019, 11, 33706–33715.
Li, I. C.; Moore, A. N.; Hartgerink, J. D. “Missing tooth” multidomain peptide nanofibers for delivery of small molecule drugs. Biomacromolecules 2016, 17, 2087–2095.
Shi, L.; Kuang, D. Q.; Ma, X. M.; Jalalah, M.; Alsareii, S. A.; Gao, T.; Harraz, F. A.; Yang, J.; Li, G. X. Peptide assembled in a nano-confined space as a molecular rectifier for the availability of ionic current modulation. Nano Lett. 2022, 22, 1083–1090.
Su, Z. Q.; Shen, H. Y.; Wang, H. X.; Wang, J. H.; Li, J. F.; Nienhaus, G. U.; Shang, L.; Wei, G. Motif-designed peptide nanofibers decorated with graphene quantum dots for simultaneous targeting and imaging of tumor cells. Adv. Funct. Mater. 2015, 25, 5472–5478.
Yang, J.; An, H. W.; Wang, H. Self-assembled peptide drug delivery systems. ACS Appl. Bio Mater. 2021, 4, 24–46.
Song, Y. L.; Wang, Y. D.; Wang, S. Y.; Cheng, Y.; Lu, Q. L.; Yang, L. F.; Tan, F. P.; Li, N. Immune-adjuvant loaded Bi2Se3 nanocage for photothermal-improved PD-L1 checkpoint blockade immune-tumor metastasis therapy. Nano Res. 2019, 12, 1770–1780.
Song, Y. Q.; Li, M. M.; Song, N.; Liu, X.; Wu, G. Y.; Zhou, H.; Long, J. F.; Shi, L. Q.; Yu, Z. L. Self-amplifying assembly of peptides in macrophages for enhanced inflammatory treatment. J. Am. Chem. Soc. 2022, 144, 6907–6917.
Pandit, G.; Roy, K.; Agarwal, U.; Chatterjee, S. Self-assembly mechanism of a peptide-based drug delivery vehicle. ACS Omega 2018, 3, 3143–3155.
Zhang, F. J.; Hu, C.; Kong, Q. S.; Luo, R. F.; Wang, Y. B. Peptide-/drug-directed self-assembly of hybrid polyurethane hydrogels for wound healing. ACS Appl. Mater. Interfaces 2019, 11, 37147–37155.
Chen, B.; He, X. Y.; Yi, X. Q.; Zhuo, R. X.; Cheng, S. X. Dual-peptide-functionalized albumin-based nanoparticles with pH-dependent self-assembly behavior for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 15148–15153.
Shen, F. Y.; Sun, L. L.; Wang, L. H.; Peng, R.; Fan, C. H.; Liu, Z. Framework nucleic acid immune adjuvant for transdermal delivery based chemo-immunotherapy for malignant melanoma treatment. Nano Lett. 2022, 22, 4509–4518.
Liu, Q.; Chen, F. Q.; Hou, L.; Shen, L. M.; Zhang, X. Q.; Wang, D. G.; Huang, L. Nanocarrier-mediated chemo-immunotherapy arrested cancer progression and induced tumor dormancy in desmoplastic melanoma. ACS Nano 2018, 12, 7812–7825.
Hong, X. Y.; Wu, Z. Z.; Chen, L. Z.; Wu, F.; Wei, L. M.; Yuan, W. E. Hydrogel microneedle arrays for transdermal drug delivery. Nano-Micro Lett. 2014, 6, 191–199.
Ramot, Y.; Haim-Zada, M.; Domb, A. J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Delivery Rev. 2016, 107, 153–162.
Demir, Y. K.; Akan, Z.; Kerimoglu, O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS One 2013, 8, e77289.
Larrañeta, E.; Lutton, R. E. M.; Woolfson, A. D.; Donnelly, R. F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32.
Qu, M. Y.; Kim, H. J.; Zhou, X. W.; Wang, C. R.; Jiang, X.; Zhu, J. X.; Xue, Y. M.; Tebon, P.; Sarabi, S. A.; Ahadian, S. et al. Biodegradable microneedle patch for transdermal gene delivery. Nanoscale 2020, 12, 16724–16729.
Wang, C.; Ye, Y. Q.; Hochu, G. M.; Sadeghifar, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PDl antibody. Nano Lett. 2016, 16, 2334–2340.
Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1444.
Tian, Y. W.; Zhang, L. M.; Liu, F. J.; Wang, M. X.; Li, L. Y.; Guo, M. M.; Xu, H. Y.; Yu, Z. Y.; Wang, W. Z. Multi-stage responsive peptide nanosensor: Anchoring EMT and mitochondria with enhanced fluorescence and boosting tumor apoptosis. Biosens. Bioelectron. 2021, 184, 113235.
Wang, Y. H.; Jia, F.; Wang, Z. H.; Qian, Y. X.; Fan, L. Y.; Gong, H.; Luo, A. Q.; Sun, J.; Hu, Z. Y.; Wang, W. Z. Boosting the theranostic effect of liposomal probes toward prominin-1 through optimized dual-site targeting. Anal. Chem. 2019, 91, 7245–7253.
Lin, X.; Lu, X.; Luo, G. S.; Xiang, H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2020, 186, 111876.
Magnez, R.; Thiroux, B.; Taront, S.; Segaoula, Z.; Quesnel, B.; Thuru, X. PD-1/PD-L1 binding studies using microscale thermophoresis. Sci. Rep. 2017, 7, 17623.
Luo, Z. M.; Sun, W. J.; Fang, J.; Lee, K.; Li, S.; Gu, Z.; Dokmeci, M. R.; Khademhosseini, A. Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery. Adv. Healthc. Mater. 2019, 8, 1801054.
Zhu, J. X.; Zhou, X. W.; Kim, H. J.; Qu, M. Y.; Jiang, X.; Lee, K.; Ren, L.; Wu, Q. Z.; Wang, C. R.; Zhu, X. M. et al. Gelatin methacryloyl microneedle patches for minimally invasive extraction of skin interstitial fluid. Small 2020, 16, 1905910.
Rodell, C. B.; Arlauckas, S. P.; Cuccarese, M. F.; Garris, C. S.; Li, R.; Ahmed, M. S.; Kohler, R. H.; Pittet, M. J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 22074006), Beijing Natural Science Foundation (No. 2222029), and Beijing Institute of Technology Research Fund Program for Young Scholars. We thank the Analysis and Testing Center in Beijing Institute of Technology on the experimental data acquisition.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Li, M., Wang, M., Li, L. et al. A composite peptide-supramolecular microneedle system for melanoma immunotherapy. Nano Res. 16, 5335–5345 (2023). https://doi.org/10.1007/s12274-022-5236-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5236-z