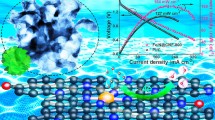
Abstract
The construction of robust coupling catalysts for accelerating electrocatalytic oxygen reduction reaction (ORR) through the modulation of the electronic structure and local atomic configuration is critical but remains challenging. Herein, we report a facile and effective isolation-polymerization-pyrolysis (IPP) strategy for high-precision synthesis of single-atomic Mn sites coupled with Fe3C nanoparticles encapsulated in N-doped porous carbon matrixes (Mn SAs/Fe3C NPs@NPC) catalyst derived from predesigned bimetallic Fe/Mn polyphthalocyanine (FeMn-BPPc) conjugated polymer networks by solid-phase reaction approach. Benefiting from the synergistic effects between the single-atomic Mn-N4 sites and Fe3C NPs as well as the confinement effect of NPC, the Mn SAs/Fe3C NPs@NPC catalyst exhibited excellent electrocatalytic activity and stability for ORR. The assembled Zn-air battery displayed larger power density of 186 mW·cm−2 than that of Pt/C + Ir/C-based battery. It also exhibits excellent stability without obvious voltage change after 106 cycles with 36 h. Combing in-situ Raman spectra with in-situ attenuated total reflectance surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) characterization results indicated that the Mn-N4 site as an active site for the O2 adsorption-activation process, which effectively facilitates the generation of key *OOH intermediates and *OH desorption to promote the multielectron reaction kinetics. Theoretical calculation reveals that the excellent electrocatalytic performance originates from the charge redistribution and the d orbital shift resulting from Mn-Fe bond, which buffers the activity of ORR through the electron reservoir capable of electron donation or releasing. This work paves a novel IPP strategy for constructing high-performance coupling electrocatalyst towards the ORR for energy conversion devices.

Similar content being viewed by others
References
Zheng, H. Z.; Ma, F.; Yang, H. C.; Wu, X. G.; Wang, R.; Jia, D. L.; Wang, Z. X.; Lu, N. D.; Ran, F.; Peng, S. L. Mn, N co-doped Co nanoparticles/porous carbon as air cathode for highly efficient rechargeable Zn-air batteries. Nano Res. 2022, 15, 1942–1948.
Zhao, Q. Q.; Butt, F. K.; Yang, M.; Guo, Z. F.; Yao, X. Y.; Zapata, M. J. M.; Zhu, Y. Q.; Ma, X. L.; Cao, C. B. Tuning oxygen redox chemistry of P2-type manganese-based oxide cathode via dual Cu and Co substitution for sodium-ion batteries. Energy Stor. Mater. 2021, 41, 581–587.
Xue, H. R.; Gong, H.; Yamauchi, Y.; Sasaki, T.; Ma, R. Z. Photoenhanced rechargeable high-energy-density metal batteries for solar energy, conversion and storage. Nano Res. Energy 2022, 1: e9120007.
Cao, Y. H.; Zhu, Y. Q.; Du, C. L.; Yang, X. Y.; Xia, T. Y.; Ma, X. L.; Cao, C. B. Anionic Te-substitution boosting the reversible redox in CuS nanosheet cathodes for magnesium storage. ACS Nano 2022, 16, 1578–1588.
Wang, Y.; Zheng, X. B.; Wang, D. S. Design concept for electrocatalysts. Nano Res. 2022, 15, 1730–1752.
Wang, Z. T.; Zhu, Y. Q.; Qiao, C.; Yang, S.; Jia, J.; Rafai, S.; Ma, X. L.; Wu, S. D.; Ji, F. Q.; Cao, C. B. Anionic Se-substitution toward high-performance CuS1−xSex nanosheet cathode for rechargeable magnesium batteries. Small 2019, 15, 1902797.
Wang, Y. Y.; Kumar, A.; Ma, M.; Jia, Y.; Wang, Y.; Zhang, Y.; Zhang, G. X.; Sun, X. M.; Yan, Z. F. Hierarchical peony-like FeCo-NC with conductive network and highly active sites as efficient electrocatalyst for rechargeable Zn-air battery. Nano Res. 2020, 13, 1090–1099.
Jing, H. Y.; Zhu, P.; Zheng, X. B.; Zhang, Z. D.; Wang, D. S.; Li, Y. D. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. 2022, 1, 100013.
Zhang, Y. X.; Zhu, Y. Q.; Wang, Z. T.; Peng, H.; Yang, X. Y.; Cao, Y. H.; Du, C. L.; Ma, X. L.; Cao, C. B. Pulverization-tolerant CuSe nanoflakes with high (110) planar orientation for high-performance magnesium storage. Adv. Funct. Mater. 2021, 31, 2104730.
Zhang, E. H.; Tao, L.; An, J. K.; Zhang, J. W.; Meng, L. Z.; Zheng, X. B.; Wang, Y.; Li, N.; Du, S. X.; Zhang, J. T. et al. Engineering the local atomic environments of indium single-atom catalysts for efficient electrochemical production of hydrogen peroxide. Angew. Chem., Int. Ed. 2022, 61, e202117347.
Zhang, N. Q.; Ye, C. L.; Yan, H.; Li, L. C.; He, H.; Wang, D. S.; Li, Y. D. Single-atom site catalysts for environmental catalysis. Nano Res. 2020, 13, 3165–3182.
Liang, Z. B.; Guo, W. H.; Zhao, R.; Qiu, T. J.; Tabassum, H.; Zou, R. Q. Engineering atomically dispersed metal sites for electrocatalytic energy conversion. Nano Energy 2019, 64, 103917.
Zhuang, Z. C.; Kang, Q.; Wang, D. S.; Li, Y. D. Single-atom catalysis enables long-life, high-energy lithium-sulfur batteries. Nano Res. 2020, 13, 1856–1866.
Qiao, M. F.; Wang, Y.; Wang, Q.; Hu, G. Z.; Mamat, X.; Zhang, S. S.; Wang, S. Y. Hierarchically ordered porous carbon with atomically dispersed FeN4 for ultraefficient oxygen reduction reaction in proton-exchange membrane fuel cells. Angew. Chem., Int. Ed. 2020, 59, 2688–2694.
Gu, J. W.; Peng, Y.; Zhou, T.; Ma, J.; Pang, H.; Yamauchi, Y. Porphyrin-based framework materials for energy conversion. Nano Res. Energy 2022, 1: e9120009.
Zhao, L.; Zhang, Y.; Huang, L. B.; Liu, X. Z.; Zhang, Q. H.; He, C.; Wu, Z. Y.; Zhang, L. J.; Wu, J. P.; Yang, W. L. et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10, 1278.
Li, J. K.; Sougrati, M. T.; Zitolo, A.; Ablett, J. M.; Oguz, I. C.; Mineva, T.; Matanovic, I.; Atanassov, P.; Huang, Y.; Zenyuk, I. et al. Identification of durable and non-durable FeNx sites in Fe-N-C materials for proton exchange membrane fuel cells. Nat. Catal. 2021, 4, 10–19.
Xiong, Y.; Sun, W. M.; Han, Y. H.; Xin, P. Y.; Zheng, X. S.; Yan, W. S.; Dong, J. C.; Zhang, J.; Wang, D. S.; Li, Y. D. Cobalt single atom site catalysts with ultrahigh metal loading for enhanced aerobic oxidation of ethylbenzene. Nano Res. 2021, 14, 2418–2423.
Chen, Z.; Liao, X. B.; Sun, C. L.; Zhao, K. N.; Ye, D. X.; Li, J. T.; Wu, G.; Fang, J. H.; Zhao, H. B.; Zhang, J. J. Enhanced performance of atomically dispersed dual-site Fe-Mn electrocatalysts through cascade reaction mechanism. Appl. Catal. B: Environ. 2021, 288, 120021.
Li, J. Z.; Chen, M. J.; Cullen, D. A.; Hwang, S.; Wang, M. Y.; Li, B. Y.; Liu, K. X.; Karakalos, S.; Lucero, M.; Zhang, H. G. et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945.
Wang, H. H.; Lv, L. B.; Zhang, S. N.; Su, H.; Zhai, G. Y.; Lei, W. W.; Li, X. H.; Chen, J. S. Synergy of Fe-N4 and non-coordinated boron atoms for highly selective oxidation of amine into nitrile. Nano Res. 2020, 13, 2079–2084.
Zhang, K.; Han, X. P.; Hu, Z.; Zhang, X. L.; Tao, Z. L.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728.
Yang, Y.; Mao, K. T.; Gao, S. Q.; Huang, H.; Xia, G. L.; Lin, Z. Y.; Jiang, P.; Wang, C. L.; Wang, H.; Chen, Q. W. O-, N-atoms-coordinated Mn cofactors within a graphene framework as bioinspired oxygen reduction reaction electrocatalysts. Adv. Mater. 2018, 30, 1801732.
Guan, J. Q.; Duan, Z. Y.; Zhang, F. X.; Kelly, S. D.; Si, R.; Dupuis, M.; Huang, Q.; Chen, J. Q.; Tang, C. H.; Li, C. Water oxidation on a mononuclear manganese heterogeneous catalyst. Nat. Catal. 2018, 1, 870–877.
Yang, Z. K.; Wang, X. L.; Zhu, M. Z.; Leng, X. Y.; Chen, W. X.; Wang, W. Y.; Xu, Q.; Yang, L. M.; Wu, Y. E. Structural revolution of atomically dispersed Mn sites dictates oxygen reduction performance. Nano Res. 2021, 14, 4512–4519.
Ye, C. W.; Xu, L. Recent advances in the design of a high performance metal-nitrogen-carbon catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2021, 9, 22218–22247.
Bai, L.; Duan, Z. Y.; Wen, X. D.; Si, R.; Guan, J. Q. Atomically dispersed manganese-based catalysts for efficient catalysis of oxygen reduction reaction. Appl. Catal. B: Environ. 2019, 257, 117930.
Zhang, J.; Zheng, C. Y.; Zhang, M. L.; Qiu, Y. J.; Xu, Q.; Cheong, W. C.; Chen, W. X.; Zheng, L. R.; Gu, L.; Hu, Z. P. et al. Controlling N-doping type in carbon to boost single-atom site Cu catalyzed transfer hydrogenation of quinoline. Nano Res. 2020, 13, 3082–3087.
Wu, K. L.; Chen, X.; Liu, S. J.; Pan, Y.; Cheong, W. C.; Zhu, W.; Cao, X.; Shen, R. A.; Chen, W. X.; Luo, J. et al. Porphyrin-like Fe-N4 sites with sulfur adjustment on hierarchical porous carbon for different rate-determining steps in oxygen reduction reaction. Nano Res. 2018, 11, 6260–6269.
Shang, H. S.; Jiang, Z. L.; Zhou, D. N.; Pei, J. J.; Wang, Y.; Dong, J. C.; Zheng, X. S.; Zhang, J. T.; Chen, W. X. Engineering a metal-organic framework derived Mn-N4-CxSy atomic interface for highly efficient oxygen reduction reaction. Chem. Sci. 2020, 11, 5994–5999.
Li, X. Y.; Rong, H. P.; Zhang, J. T.; Wang, D. S.; Li, Y. D. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 2020, 13, 1842–1855.
Xu, J.; Lai, S. H.; Qi, D. F.; Hu, M.; Peng, X. Y.; Liu, Y. F.; Liu, W.; Hu, G. Z.; Xu, H.; Li, F. et al. Atomic Fe-Zn dual-metal sites for high-efficiency pH-universal oxygen reduction catalysis. Nano Res. 2021, 14, 1374–1381.
Han, A. L.; Wang, X. J.; Tang, K.; Zhang, Z. D.; Ye, C. L.; Kong, K. J.; Hu, H. B.; Zheng, L. R.; Jiang, P.; Zhao, C. X. et al. An adjacent atomic platinum site enables single-atom iron with high oxygen reduction reaction performance. Angew. Chem., Int. Ed. 2021, 60, 19262–19271.
Yin, P. Q.; Yao, T.; Wu, Y. E.; Zheng, L. R.; Lin, Y.; Liu, W.; Ju, H. X.; Zhu, J. F.; Hong, X.; Deng, Z. X. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem., Int. Ed. 2016, 55, 10800–10805.
Xiao, G. F.; Lu, R. H.; Liu, J. F.; Liao, X. B.; Wang, Z. Y.; Zhao, Y. Coordination environments tune the activity of oxygen catalysis on single atom catalysts: A computational study. Nano Res. 2022, 15, 3073–3081.
Wang, J.; Li, Z. J.; Wu, Y. E.; Li, Y. D. Fabrication of single-atom catalysts with precise structure and high metal loading. Adv. Mater. 2018, 30, 1801649.
Khani, H.; Grundish, N. S.; Wipf, D. O.; Goodenough, J. B. Graphitic-shell encapsulation of metal electrocatalysts for oxygen evolution, oxygen reduction, and hydrogen evolution in alkaline solution. Adv. Energy Mater. 2020, 10, 1903215.
Liu, X.; Liu, H.; Chen, C.; Zou, L. L.; Li, Y.; Zhang, Q.; Yang, B.; Zou, Z. Q.; Yang, H. Fe2N nanoparticles boosting FeNx moieties for highly efficient oxygen reduction reaction in Fe-N-C porous catalyst. Nano Res. 2019, 12, 1651–1657.
Li, Y. Z.; Cao, R.; Li, L. B.; Tang, X. N.; Chu, T. L.; Huang, B. Y.; Yuan, K.; Chen, Y. W. Simultaneously integrating single atomic cobalt sites and Co9S8 nanoparticles into hollow carbon nanotubes as trifunctional electrocatalysts for Zn-air batteries to drive water splitting. Small 2020, 16, 1906735.
Meng, L. X.; Li, L. Recent research progress on operational stability of metal oxide/sulfide photoanodes in photoelectrochemical cells. Nano Res. Energy, 2022, https://doi.org/10.1016/26599/NRE.2022.9120020.
Wei, X. Q.; Song, S. J.; Wu, N. N.; Luo, X.; Zheng, L. R.; Jiao, L.; Wang, H. J.; Fang, Q.; Hu, L. Y.; Gu, W. L. et al. Synergistically enhanced single-atomic site Fe by Fe3C@C for boosted oxygen reduction in neutral electrolyte. Nano Energy 2021, 84, 105840.
Zhou, F. L.; Yu, P.; Sun, F. F.; Zhang, G. Y.; Liu, X.; Wang, L. The cooperation of Fe3C nanoparticles with isolated single iron atoms to boost the oxygen reduction reaction for Zn-air batteries. J. Mater. Chem. A 2021, 9, 6831–6840.
Cui, X.; Gao, L. K.; Lei, S.; Liang, S.; Zhang, J. W.; Sewell, C. D.; Xue, W. D.; Liu, D.; Lin, Z. Q.; Yang, Y. K. Simultaneously crafting single-atomic Fe sites and graphitic layer-wrapped Fe3C nanoparticles encapsulated within mesoporous carbon tubes for oxygen reduction. Adv. Funct. Mater. 2021, 31, 2009197.
Kang, G. S.; Jang, J. H.; Son, S. Y.; Lee, C. H.; Lee, Y. K.; Lee, D. C.; Yoo, S. J.; Lee, S.; Joh, H. I. Fe-based non-noble metal catalysts with dual active sites of nanosized metal carbide and single-atomic species for oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 22379–22388.
Kong, F. T.; Fan, X. H.; Kong, A. G.; Zhou, Z. Q.; Zhang, X. Y.; Shan, Y. K. Covalent phenanthroline framework derived FeS@Fe3C composite nanoparticles embedding in N-S-codoped carbons as highly efficient trifunctional electrocatalysts. Adv. Funct. Mater. 2018, 28, 1803973.
Ren, G. Y.; Lu, X. Y.; Li, Y. A.; Zhu, Y.; Dai, L.; Jiang, L. Porous core-shell Fe3C embedded N-doped carbon nanofibers as an effective electrocatalysts for oxygen reduction reaction. ACS Appl. Mater. Inter. 2016, 8, 4118–4125.
Yang, C. C.; Zai, S. F.; Zhou, Y. T.; Du, L.; Jiang, Q. Fe3C-Co nanoparticles encapsulated in a hierarchical structure of N-doped carbon as a multifunctional electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949.
Feng, X. Q.; Guo, J. H.; Wang, S. R.; Wu, Q. K.; Chen, Z. Atomically dispersed gold anchored on carbon nitride nanosheets as effective catalyst for regioselective hydrosilylation of alkynes. J. Mater. Chem. A 2021, 9, 17885–17892.
Guan, J. Q.; Bai, X.; Tang, T. M. Recent progress and prospect of carbon-free single-site catalysts for the hydrogen and oxygen evolution reactions. Nano Res. 2022, 15, 818–837.
Jiang, H.; Gu, J. X.; Zheng, X. S.; Liu, M.; Qiu, X. Q.; Wang, L. B.; Li, W. Z.; Chen, Z. F.; Ji, X. B.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333.
Hu, B. T.; Huang, A. J.; Zhang, X. J.; Chen, Z.; Tu, R. Y.; Zhu, W.; Zhuang, Z. B.; Chen, C.; Peng, Q.; Li, Y. D. Atomic Co/Ni dual sites with N/P-coordination as bifunctional oxygen electrocatalyst for rechargeable zinc-air batteries. Nano Res. 2021, 14, 3482–3488.
Wang, L. Q.; Han, Z. L.; Zhao, Q. Q.; Yao, X. Y.; Zhu, Y. Q.; Ma, X. L.; Wu, S. D.; Cao, C. B. Engineering yolk-shell P-doped NiS2/C spheres via a MOF-template for high-performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 8612–8619.
Wang, S. R.; Wang, M. M.; Liu, Z.; Liu, S. J.; Chen, Y. J.; Li, M.; Zhang, H.; Wu, Q. K.; Guo, J. H.; Feng, X. Q. et al. Synergetic function of the single-atom Ru-N4 site and Ru nanoparticles for hydrogen production in a wide pH range and seawater electrolysis. ACS Appl. Mater. Interfaces 2022, 14, 15250–15258.
Chen, Y. J.; Gao, R.; Ji, S. F.; Li, H. J.; Tang, K.; Jiang, P.; Hu, H. B.; Zhang, Z. D.; Hao, H. G.; Qu, Q. Y. et al. Atomic-level modulation of electronic density at cobalt single-atom sites derived from metal-organic frameworks: Enhanced oxygen reduction performance. Angew. Chem., Int. Ed. 2021, 60, 3212–3221.
Cui, T. T.; Wang, Y. P.; Ye, T.; Wu, J.; Chen, Z. Q.; Li, J.; Lei, Y. P.; Wang, D. S.; Li, Y. D. Engineering dual single-atom sites on 2D ultrathin N-doped carbon nanosheets attaining ultra-low-temperature zinc-air battery. Angew. Chem., Int. Ed. 2022, 61, e202115219.
Li, L. L.; Hasan, I. M.; He, R. N.; Peng, L. W.; Xu, N. N.; N, N. K.; Zhang, J. -N.; Qiao, J. L. Copper as a single metal atom based photo-, electro- and photoelectrochemical catalyst decorated on carbon nitride surface for efficient CO2 reduction: A review. Nano Res. Energy, 2022, https://doi.org/10.26599/NRE.2022.9120015.
Liang, J.; Liu, Q.; Alshehri, A. A.; Sun X. P. Recent advances in nanostructured heterogeneous catalysts for N-cycle electrocatalysis. Nano Res. Energy, 2022, https://doi.org/10.1016/26599/NRE.2022.9120010.
Chen, Z.; Yang, W. J.; Wu, Y.; Zhang, C.; Luo, J.; Chen, C.; Li, Y. D. Atomic iron on mesoporous N-doped carbon to achieve dehydrogenation reaction at room temperature. Nano Res. 2020, 13, 3075–3081.
Acknowledgements
This work was supported by State Key Laboratory of Catalytic Materials and Reaction Engineering (RIPP, SINOPEC), Taishan Scholars Program of Shandong Province (No. tsqn201909065), Shandong Provincial Natural Science Foundation (Nos. ZR2021YQ15, ZR2020QB174, and ZR2019MB022), the National Natural Science Foundation of China (Nos. 22108306 and 21902182), the Fundamental Research Funds for the Central Universities (Nos. 2022YQHH01 and 22CX07009A), the State Key Laboratory of Organic-Inorganic Composites (No. oic-202101006), Post-graduate Innovation Fund of China University of Petroleum (East China) (No. YCX2021064), the Research Fund Program of Key Laboratory of Fuel Cell Technology of Guangdong Province, the Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), and the Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4502_MOESM1_ESM.pdf
Single-atomic Mn sites coupled with Fe3C nanoparticles encapsulated in carbon matrixes derived from bimetallic Mn/Fe polyphthalocyanine conjugated polymer networks for accelerating electrocatalytic oxygen reduction
Rights and permissions
About this article
Cite this article
Pan, Y., Li, M., Mi, W. et al. Single-atomic Mn sites coupled with Fe3C nanoparticles encapsulated in carbon matrixes derived from bimetallic Mn/Fe polyphthalocyanine conjugated polymer networks for accelerating electrocatalytic oxygen reduction. Nano Res. 15, 7976–7985 (2022). https://doi.org/10.1007/s12274-022-4502-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4502-4