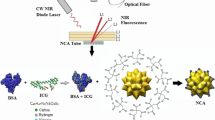
Abstract
One of the thorny problems currently impeding the applications of the fluorescence imaging technique is the poor spatial resolution in deep tissue. Ultrasound-switchable fluorescence (USF) imaging is a novel imaging tool that has recently been explored to possibly surmount the above-mentioned bottleneck. Herein, a β-cyclodextrin/indocyanine green (ICG) complex-encapsulated poly(N-isopropylacrylamide) (PNIPAM) nanogel was synthesized and studied for ex vivo/in vivo deep tissue/high-resolution near infrared USF (NIR-USF) imaging. To be specific, our results revealed that the average diameter of the as-prepared nanogels was significantly decreased to ~ 32 nm from ~ 335 nm compared to the reported ICG-PNIPAM nanoparticles. Additionally, the excitation/ emission characteristics of the ICG itself in present nanogels were almost completely retained, and the resultant nanogel exhibited high physiological stability and positive biocompatibility. In particular, the signal-to-noise ratio of the USF image for the PNIPAM/ β-cyclodextrin/ICG nanogel (33.01 ± 2.42 dB) was prominently higher than that of the ICG-PNIPAM nanoparticles (18.73 ± 0.33 dB) in 1.5-cm-thick chicken breast tissues. The NIR-USF imaging in 3.5-cm-thick chicken breast tissues was achieved using this new probe. The ex vivo NIR-USF imaging of the mouse liver was also successfully obtained. Animal experiments showed that the present nanogels were able to be effectively accumulated into U87 tumor-bearing mice via enhanced permeability and retention effects, and the high-resolution NIR-USF imaging of in vivo tumor was efficiently acquired. The metabolism and in vivo biodistribution of the nanogels were evaluated. Overall, the results suggest that the current nanogel is a highly promising NIR-USF probe for deep tissue and high-resolution USF imaging.

Similar content being viewed by others
References
Shou, K. Q.; Qu, C. R.; Sun, Y.; Chen, H.; Chen, S.; Zhang, L.; Xu, H. B.; Hong, X. C.; Yu, A. X.; Cheng, Z. Multifunctional biomedical imaging in physiological and pathological conditions using a NIR-II probe. Adv. Funct. Mater.2017, 27, 1700995.
Carr, J. A.; Franke, D.; Caram, J. R.; Perkinson, C. F.; Saif, M.; Askoxylakis, V.; Datta, M.; Fukumura, D.; Jain, R. K.; Bawendi, M. G. et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl. Acad. Sci. USA2018, 115, 4465–4470.
Hong, G. S.; Antaris, A. L.; Dai, H. J. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng.2017, 1, 0010.
Qi, J.; Sun, C. W.; Li, D. Y.; Zhang, H. Q.; Yu, W. B.; Zebibula, A.; Lam, J. W. Y.; Xi, W.; Zhu, L.; Cai, F. H. et al. Aggregation-induced emission luminogen with near-infrared-II excitation and nearinfrared- I emission for ultradeep intravital two-photon microscopy. ACS Nano2018, 12, 7936–7945.
Frangioni, J. V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol.2003, 7, 626–634.
Yuan, B. H.; Liu, Y.; Mehl, P. M.; Vignola, J. Microbubble-enhanced ultrasound-modulated fluorescence in a turbid medium. Appl. Phys. Lett.2009, 95, 181113.
Huynh, N. T.; Hayes-Gill, B. R.; Zhang, F.; Morgan, S. P. Ultrasound modulated imaging of luminescence generated within a scattering medium. J. Biomed. Opt.2013, 18, 20505.
Lin, Y. T.; Bolisay, L. D.; Ghijsen, M.; Kwong, T. C.; Gulsen, G. Temperature-modulated fluorescence tomography in a turbid media. Appl. Phys. Lett.2012, 100, 073702.
Lin, Y. T.; Kwong, T. C.; Bolisay, L.; Gulsen, G. Temperaturemodulated fluorescence tomography based on both concentration and lifetime contrast. J. Biomed. Opt.2012, 17, 056007.
Yuan, B. H.; Uchiyama, S.; Liu, Y.; Nguyen, K. T.; Alexandrakis, G. -resolution imaging in a deep turbid medium based on an ultrasound-switchable fluorescence technique. Appl. Phys. Lett.2012, 101, 033703.
Ahmad, J.; Jayet, B.; Hill, P. J.; Mather, M. L.; Dehghani, H.; Morgan, S. P. Ultrasound-mediation of self-illuminating reporters improves imaging resolution in optically scattering media. Biomed. Opt. Express2018, 9, 1664–1679.
Xu, X.; Liu, H. L.; Wang, L. V. Time-reversed ultrasonically encoded optical focusing into scattering media. Nat. Photonics2011, 5, 154–157.
Yu, S.; Cheng, B. B.; Yao, T. F.; Xu, C. C.; Nguyen, K. T.; Hong, Y.; Yuan, B. H. New generation ICG-based contrast agents for ultrasound-switchable fluorescence imaging. Sci. Rep.2016, 6, 35942.
Kwong, T. C. A new modality for high resolution diffuse optical imaging: Temperature modulated fluorescence tomography. Ph.D. Dissertation, University of California, Irvine, USA, 2017.
Yao, T. F.; Yu, S.; Liu, Y.; Yuan, B. H. Ultrasound-switchable fluorescence imaging via an EMCCD camera and a Z-scan method. IEEE J. Sel. Top. Quantum Electron.2019, 25, 7102108.
Yao, T. F.; Yu, S.; Liu, Y.; Yuan, B. H. In vivo ultrasound-switchable fluorescence imaging. Sci. Rep.2019, 9, 9855.
Pei, Y. B.; Wei, M. Y.; Cheng, B. B.; Liu, Y.; Xie, Z. W.; Nguyen, K.; Yuan, B. H. High resolution imaging beyond the acoustic diffraction limit in deep tissue via ultrasound-switchable NIR fluorescence. Sci. Rep.2014, 4, 4690.
Rodriguez, V. B.; Henry, S. M.; Hoffman, A. S.; Stayton, P. S.; Li, X. D.; Pun, S. H. Encapsulation and stabilization of indocyanine green within poly(styrene-alt-maleic anhydride) block-poly(styrene) micelles for near-infrared imaging. J. Biomed. Opt.2008, 13, 014025.
Shan, W. J.; Chen, R. H.; Zhang, Q.; Zhao, J.; Chen, B. B.; Zhou, X.; Ye, S. F.; Bi, S. L.; Nie, L. M.; Ren, L. Improved stable indocyanine green (ICG)-mediated cancer optotheranostics with naturalized hepatitis B core particles. Adv. Mater.2018, 30, 1707567.
Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci.2003, 92, 2090–2097.
Holzer, W.; Mauerer, M.; Penzkofer, A.; Szeimies, R. M.; Abels, C.; Landthaler, M.; Bäumler, W. Photostability and thermal stability of indocyanine green. J. Photochem. Photobiol. B1998, 47, 155–164.
Jambhekar, S. S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discovery Today2016, 21, 363–368.
Liu, R. L.; Zhang, Z. Q.; Jing, W. H.; Wang, L.; Luo, Z. M.; Chang, R. M.; Zeng, A. G.; Du, W.; Chang, C.; Fu, Q. β-Cyclodextrin anchoring onto pericarpium granati-derived magnetic mesoporous carbon for selective capture of lopid in human serum and pharmaceutical wastewater samples. Mater. Sci. Eng. C2016, 62, 605–613.
Barros, T. C.; Toma, S. H.; Toma, H. E.; Bastos, E. L.; Baptista, M. S. Polymethine cyanine dyes in β-cyclodextrin solution: Multiple equilibria and chemical oxidation. J. Phys. Org. Chem.2010, 23, 893–903.
DeDora, D. J.; Suhrland, C.; Goenka, S.; Chowdhury, S. M.; Lalwani, G.; Mujica-Parodi, L. R.; Sitharaman, B. Sulfobutyl ether β-cyclodextrin (Captisol®) and methyl β-cyclodextrin enhance and stabilize fluorescence of aqueous indocyanine green. J. Biomed. Mater. Res. Part B: Appl. Biomater.2016, 104, 1457–1464.
Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr.1991, 54, 1119S–1124S.
Liu, R. L.; Wang, Y.; Ge, X. L.; Yu, P.; Liu, H. Q.; Wang, M. C.; Lu, W.; Fu, Q. Polydopamine/polyethyleneimine complex adhered to micrometer-sized magnetic carbon fibers for high-efficiency hemoperfusion. J. Biomater. Sci. Polym. Ed.2017, 28, 1444–1468.
Blackburn, W. H.; Andrew Lyon, L. Size-controlled synthesis of monodisperse core/shell nanogels. Colloid Polym. Sci.2008, 286, 563–569.
Freitag, R.; Garret-Flaudy, F. Salt effects on the thermoprecipitation of poly-(N-isopropylacrylamide) oligomers from aqueous solution. Langmuir2002, 18, 3434–3440.
Dai, Y. L.; Xu, C.; Sun, X. L.; Chen, X. Y. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev.2017, 46, 3830–3852.
Bhavane, R.; Starosolski, Z.; Stupin, I.; Ghaghada, K. B.; Annapragada, A. NIR-II fuorescence imaging using indocyanine green nanoparticles. Sci. Rep.2018, 8, 14455.
Zhu, Q. S.; Chen, L. B.; Zhu, P. Y.; Luan, J. F.; Mao, C.; Huang, X. H.; Shen, J. Preparation of PNIPAM-g-P (NIPAM-co-St) microspheres and their blood compatibility. Colloids Surf. B: Biointerfaces2013, 104, 61–65.
Cheng, B. B.; Bandi, V.; Wei, M. Y.; Pei, Y. B.; D’Souza, F.; Nguyen, K. T.; Hong, Y.; Yuan, B. H. High-resolution ultrasound-switchable fluorescence imaging in centimeter-deep tissue phantoms with high signal-to-noise ratio and high sensitivity via novel contrast agents. PLoS One2016, 11, e0165963.
Zhao, J. Y.; Zhong, D.; Zhou, S. B. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J. Mater. Chem. B2018, 6, 349–365.
Yuan, A.; Wu, J. H.; Tang, X. L.; Zhao, L. L.; Xu, F.; Hu, Y. Q. Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies. J. Pharm. Sci.2013, 102, 6–28.
Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods2008, 5, 763–775.
Hong, G. S.; Lee, J. C.; Robinson, J. T.; Raaz, U.; Xie, L. M.; Huang, N. F.; Cooke, J. P.; Dai, H. J. Multifunctional In vivo vascular imaging using near-infrared II fluorescence. Nat. Med.2012, 18, 1841–1846.
Bhavane, R.; Starosolski, Z.; Stupin, I.; Ghaghada, K. B.; Annapragada, A. NIR-II fluorescence imaging using indocyanine green nanoparticles. Sci. Rep.2018, 8, 14455.
Wang, Y. F.; Liu, G. Y.; Sun, L. D.; Xiao, J. W.; Zhou, J. C.; Yan, C. H. Nd3+-sensitized upconversion nanophosphors: Efficient In vivo bioimaging probes with minimized heating effect. ACS Nano2013, 7, 7200–7206.
Chen, G. Y.; Shen, J.; Ohulchanskyy, T. Y.; Patel, N. J.; Kutikov, A.; Li, Z. P.; Song, J.; Pandey, R. K.; Ågren, H.; Prasad, P. N. et al. (α-NaYbF4:Tm3+)/CaF2 core/shell nanoparticles with efficient nearinfrared to near-infrared upconversion for high-contrast deep tissue bioimaging. ACS Nano2012, 6, 8280–8287.
Yu, M. X.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano2015, 9, 6655–6674.
Imamura, T.; Saitou, T.; Kawakami, R. In vivo optical imaging of cancer cell function and tumor microenvironment. Cancer Sci.2018, 109, 912–918.
Vanparijs, N.; Nuhn, L.; De Geest, B. G. Transiently thermoresponsive polymers and their applications in biomedicine. Chem. Soc. Rev.2017, 46, 1193–1239.
Zeng, Y.; Zhu, J.; Wang, J. Q.; Parasuraman, P.; Busi, S.; Nauli, S. M.; Wáng, Y. X. J.; Pala, R.; Liu, G. Functional probes for cardiovascular molecular imaging. Quant. Imaging Med. Surg.2018, 8, 838–852.
Acknowledgements
This work was supported in part by funding from the CPRIT RP170564 (Baohong Yuan) and the NSF CBET-1253199 (Baohong Yuan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal studies were approved by the University of Texas at Arlington’s Institutional Animal Care and Use Committee and performed in accordance with their guidance and regulations.
Electronic supplementary material
12274_2020_2752_MOESM1_ESM.pdf
Temperature-sensitive polymeric nanogels encapsulating with β-cyclodextrin and ICG complex for high-resolution deep-tissue ultrasound-switchable fluorescence imaging
Rights and permissions
About this article
Cite this article
Liu, R., Yao, T., Liu, Y. et al. Temperature-sensitive polymeric nanogels encapsulating with β-cyclodextrin and ICG complex for high-resolution deep-tissue ultrasound-switchable fluorescence imaging. Nano Res. 13, 1100–1110 (2020). https://doi.org/10.1007/s12274-020-2752-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2752-6