Abstract
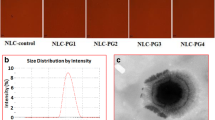
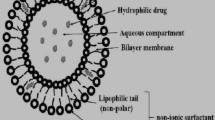
The aim of this study was to develop a ginsenoside-modified nanostructured lipid carrier (G-NLC) dispersion containing curcumin. The NLC was prepared by melt emulsification with slight modification process. Different G-NLC dispersion systems were prepared using lipid carrier matrix composed of ginsenoside, phosphatidylcholine, lysophosphatidylcholine, and hydrogenated bean oil. TEM image of the nanoparticles in the NLC dispersion showed core/shell structure, and there was corona-like layer surrounding the particles in the G-NLC. The mean particle size of G-NLC dispersion was in the range of about 300–500 nm and stayed submicron size up to 12 months. The in vitro release of curcumin was faster in pH 1.2 compared to pH 6.8 and it showed linear release pattern after lag time of 1 h. When the G-NLC dispersion was orally administered to rats, Cmax of the free curcumin was 15.2 and 32.3 ng/mL at doses of 50 and 100 mg/kg, respectively, while it was below quantification limit when curcumin was administered as of dispersion in distilled water. Based on these results, it is certain that ginsenoside modulated the NLC dispersion, leading to enduring shelf-life of the dispersion system and enhanced bioavailability. These results strongly suggest that ginsenoside holds a promising potential as a pharmaceutical excipient in the pharmaceutical industries to increase the utility of various bioactives.






Similar content being viewed by others
References
Anuchapreeda S, Fukumori Y, Okonogi S, Ichikawa H (2012) Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. J Nanotechnol 41:1–11
Arun G, Shweta P, Upendra KJ (2012) Formulation and evaluation of ternary solid dispersion of curcumin. Int J Pharm Pharm Sci 4:360–365
Asai A, Miyazawa T (2000) Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci 67:2785–2793
Baskaran R, Madheswaran T, Sundaramoorthy P, Kim HM, Yoo BK (2014) Entrapment of curcumin into monoolein-based liquid crystalline nanoparticle dispersion for enhancement of stability and anticancer activity. Int J Nanomed 9:3119–3130
Byeon SE, Choi WS, Hong EK, Lee J, Rhee MH, Park HJ, Cho JY (2009) Inhibitory effect of saponin fraction from codonopsis lanceolata on immune cell-mediated inflammatory responses. Arch Pharm Res 32:813–822
Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ, Togni S, Dixon BM (2011) Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod 74:664–669
Du ZY, Wei X, Huang MT, Zheng X, Liu Y, Conney AH, Zhang K (2013) Anti-proliferative, anti-inflammatory and antioxidant effects of curcumin analogue A2. Arch Pharm Res 36:1204–1210
Goel A, Aggarwal BB (2010) Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer 62:919–930
Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG (2010) Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem 58:2095–2099
Greenwood R, Kendall K (1990) Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Discuss Chem Soc 90:301–312
Hanaor DAH, Michelazzi M, Leonelli C, Sorrell CC (2012) The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J Eur Ceram Soc 32:235–244
Karunagaran D, Rashmi R, Kumar TR (2005) Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets 5:117–129
Khalil NM, do Nascimento TC, Casa DM, Dalmolin LF, de Mattos AC, Hoss I, Romano MA, Mainardes RM (2013) Pharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces 101:353–360
Kim JS, Joo EJ, Chun J, Ha WW, Lee JH, Han Y, Kim YS (2012) Induction of apoptosis by ginsenoside Rk1 in SK-MEL-2-human melanoma. Arch Pharm Res 35:717–722
Kulkarni S, Dhir A (2010) An overview of curcumin in neurological disorders. Indian J Pharm Sci 72:149–154
Kumar SS, Mahesh A, Mahadevan S, Mandal AB (2014) Synthesis and characterization of curcumin loaded polymer/lipid based nanoparticles and evaluation of their antitumor effects on MCF-7 cells. Biochim Biophys Acta 1840:1913–1922
Madheswaran T, Baskaran R, Sundaramoorthy P, Yoo BK (2015) Enhanced skin permeation of 5α-reductase inhibitors entrapped into surface-modified liquid crystalline nanoparticles. Arch Pharm Res 38:534–542
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK (2007) Curcumin–phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm 330:155–163
Menon VP, Sudheer AR (2007) Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 595:105–125
Piao XL, Wu Q, Yang J, Park SY, Chen DJ, Liu HM (2013) Dammarane-type saponins from heat-processed Gynostemma pentaphyllum show fortified activity against A549 cells. Arch Pharm Res 36:874–879
Popat A, Karmakar S, Jambhrunkar S, Xu C, Yu C (2014) Curcumin-cyclodextrin encapsulated chitosan nanoconjugates with enhanced solubility and cell cytotoxicity. Colloids Surf B Biointerfaces 117:520–527
Prasad S, Tyagi AK, Aggarwal BB (2014) Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 46:2–18
Ravindran J, Prasad S, Aggarwal BB (2009) Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J 11:495–510
Sinha R, Anderson DE, McDonald SS, Greenwald P (2003) Cancer risk and diet in India. J Postgrad Med 49:222–228
Tabatabaei Mirakabad FS, Akbarzadeh A, Milani M, Zarghami N, Taheri-Anganeh M, Zeighamian V, Badrzadeh F, Rahmati-Yamchi M (2016) A comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif Cells Nanomed Biotechnol 44:423–430
Udompornmongkol P, Chiang BH (2015) Curcumin-loaded polymeric nanoparticles for enhanced anti-colorectal cancer applications. J Biomater Appl 30:537–546
Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE (2008) Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomark Prev 17:1411–1417
Vijayakumar A, Baskaran R, Jang YS, Oh SH, Yoo BK (2017) Quercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. AAPS PharmSciTech 18:875–883
Wang W, Zhu R, Xie Q, Li A, Xiao Y, Li K, Liu H, Cui D, Chen Y, Wang S (2012) Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomed 7:3667–3677
Zhang YX, Wang L, Xiao EL, Li SJ, Chen JJ, Gao B, Min GN, Wang ZP, Wu YJ (2013) Ginsenoside-Rd exhibits anti-inflammatory activities through elevation of antioxidant enzyme activities and inhibition of JNK and ERK activation in vivo. Int Immunopharmacol 17:1094–1100
Acknowledgements
This study was supported by the Research Center Hospital Project in Gil Hospital, Gachon University (FRD2014-06-02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Vijayakumar, A., Baskaran, R., Maeng, HJ. et al. Ginsenoside improves physicochemical properties and bioavailability of curcumin-loaded nanostructured lipid carrier. Arch. Pharm. Res. 40, 864–874 (2017). https://doi.org/10.1007/s12272-017-0930-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0930-1