Abstract
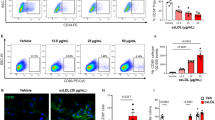
Tetrahydrobiopterin (BH4) has been known to be an essential cofactor for the activities of nitric oxide (NO) synthase and aromatic amino acid hydroxylases, which are involved in physiological and pathological processes. In the present study, we report that sepiapterin, the more stable form of BH4 precursor, modulates vascular endothelial growth factor-A (VEGF-A)-induced cell proliferation and adhesion in human umbilical vein endothelial cells (HUVECs). The antiproliferative activity of sepiapterin in VEGF-A-treated HUVECs is associated with inhibition of the expression of cyclin-dependent kinases (Cdks) such as Cdk4 and Cdk2. Pretreatment with NO synthase inhibitor does not abrogate the ability of sepiapterin to inhibit VEGF-A-induced cell proliferation and adhesion, indicating that the suppressive effects of sepiapterin on VEGF-Ainduced responses are mediated by NO-independent mechanism. Finally, we show that sepiapterin modulates VEGF-A-induced cell proliferation and adhesion through down-regulation of VEGF receptor-2 downstream signaling pathways. Taken together, these findings represent a novel function of sepiapterin in the regulation of angiogenesis, supporting further development and evaluation of sepiapterin as an antiangiogenic agent.
Similar content being viewed by others
References
Alper, O., Bergmann-Leitner, E. S., Bennett, T. A., Hacker, N. F., Stromberg, K., and Stetler-Stevenson, W. G., Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. J. Natl. Cancer Inst., 93, 1375–1384 (2001).
Baker, A. H., Edwards, D. R., and Murphy, G., Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci., 115, 3719–3727 (2002).
Bourboulia, D. and Stetler-Stevenson, W. G., Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin. Cancer Biol., 20, 161–168 (2010).
Brew, K. and Nagase, H., The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta, 1803, 55–71 (2010).
Carmeliet, P. and Jain, R. K., Angiogenesis in cancer and other diseases. Nature, 407, 249–257 (2000).
Chen, L., Zeng, X., Wang, J., Briggs, S. S., O’Neill, E., Li, J., Leek, R., Kerr, D. J., Harris, A. L., and Cai, S., Roles of tetrahydrobiopterin in promoting tumor angiogenesis. Am. J. Pathol., 177, 2671–2680 (2010).
Cho, Y. R., Kim, S. H., Ko, H. Y., Kim, M. D., Choi, S. W., and Seo, D. W., Sepiapterin inhibits cell proliferation and migration of ovarian cancer cells via down-regulation of p70S6K-dependent VEGFR-2 expression. Oncol. Rep., 26, 861–867 (2011).
Christofori, G., New signals from the invasive front. Nature, 441, 444–450 (2006).
Ferrara, N., Gerber, H. P., and LeCouter, J., The biology of VEGF and its receptors. Nat. Med., 9, 669–676 (2003).
Folkman, J., Angiogenesis. Annu. Rev. Med., 57, 1–18 (2006).
Gao, L., Pung, Y. F., Zhang, J., Chen, P., Wang, T., Li, M., Meza, M., Toro, L., and Cai, H., Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol., 297, H331–H339 (2009).
Gumbiner, B. M., Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell, 84, 345–357 (1996).
Holmes, K., Roberts, O. L., Thomas, A. M., and Cross, M. J., Vascular endothelial growth factor receptor-2: structure, function, intracellular signaling and therapeutic inhibition. Cell. Signal., 19, 2003–2012 (2007).
Jiang, X., Kim, B., Lin, H., Lee, C. K., Kim, J., Kang, H., Lee, P., Jung, S. H., Lee, H. M., and Won, K. J., Tetrahydrobiopterin inhibits PDGF-stimulated migration and proliferation in rat aortic smooth muscle cells via the nitric oxide synthase-independent pathway. Korean J. Physiol. Pharmacol., 14, 177–183 (2010).
Katusic, Z. S., d’Uscio, L. V., and Nath, K. A., Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol. Sci., 30, 48–54 (2009).
Kimura, H., Weisz, A., Kurashima, Y., Hashimoto, K., Ogura, T., D’Acquisto, F., Addeo, R., Makuuchi, M., and Esumi, H., Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood, 95, 189–197 (2000).
Lamy, S., Lachambre, M. P., Lord-Dufour, S., and Beliveau, R., Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul. Pharmacol., 53, 200–208 (2010).
Malumbres, M. and Barbacid, M., Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer, 9, 153–166 (2009).
Milkiewicz, M., Hudlicka, O., Brown, M. D., and Silgram, H., Nitric oxide, VEGF, and VEGFR-2: interactions in activityinduced angiogenesis in rat skeletal muscle. Am. J. Physiol. Heart Circ. Physiol., 289, H336–H343 (2005).
Oh, J., Seo, D. W., Diaz, T., Wei, B., Ward, Y., Ray, J. M., Morioka, Y., Shi, S., Kitayama, H., Takahashi, C., Noda, M., and Stetler-Stevenson, W. G., Tissue inhibitors of metalloproteinase 2 inhibits endothelial cell migration through increased expression of RECK. Cancer Res., 64, 9062–9069 (2004).
Pannirselvam, M., Wiehler, W. B., Anderson, T., and Triggle, C. R., Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br. J. Pharmacol., 144, 953–960 (2005).
Seo, D. W., Li, H., Guedez, L., Wingfield, P. T., Diaz, T., Salloum, R., Wei, B. Y., and Stetler-Stevenson, W. G., TIMP-2 mediated inhibition of angiogenesis: an MMPindependent mechanism. Cell, 114, 171–180 (2003).
Seo, D. W., Li, H., Qu, C. K., Oh, J., Kim, Y. S., Diaz, T., Wei, B., Han, J. W., and Stetler-Stevenson, W. G., Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J. Biol. Chem., 281, 3711–3721 (2006).
Seo, D. W., Kim, S. H., Eom, S. H., Yoon, H. J., Cho, Y. R., Kim, P. H., Kim, Y. K., Han, J. W., Diaz, T., Wei, B. Y., and Stetler-Stevenson, W. G., TIMP-2 disrupts FGF-2-induced downstream signaling pathways. Microvasc. Res., 76, 145–151 (2008).
Spannuth, W. A., Sood, A. K., and Coleman, R. L., Angiogenesis as a strategic target for ovarian cancer therapy. Nat. Clin. Pract. Oncol., 5, 194–204 (2008).
Stetler-Stevenson, W. G., and Seo, D. W., TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol. Med., 11, 97–103 (2005).
Stetler-Stevenson, W. G., Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci. Signal., 1, re6 (2008).
Thony, B., Auerbach, G., and Blau, N., Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J., 347 Pt1, 1–16 (2000).
Wingfield, P. T., Sax, J. K., Stahl, S. J., Kaufman, J., Palmer, I., Chung, V., Corcoran, M. L., Kleiner, D. E., and Stetler-Stevenson, W. G., Biophysical and functional characterization of full-length, recombinant human tissue inhibitor of metalloproteinases-2 (TIMP-2) produced in Escherichia coli. Comparison of wild type and amino-terminal alanine appended variant with implications for the mechanism of TIMP functions. J. Biol. Chem., 274, 21362–21368 (1999).
Ziche, M., Morbidelli, L., Choudhuri, R., Zhang, H. T., Donnini, S., Granger, H. J., and Bicknell, R., Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factorinduced angiogenesis. J. Clin. Invest., 99, 2625–2634 (1997).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, S.H., Cho, YR., Kim, MD. et al. Inhibitory effects of sepiapterin on vascular endothelial growth factor-a-induced proliferation and adhesion in human umbilical vein endothelial cells. Arch. Pharm. Res. 34, 1571–1577 (2011). https://doi.org/10.1007/s12272-011-0920-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-011-0920-7