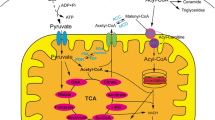
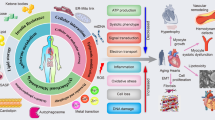
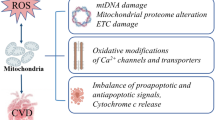
Abstract
Cardiovascular diseases (CVD) account for the largest bulk of deaths worldwide, posing a massive burden on societies and the global healthcare system. Besides, the incidence and prevalence of these diseases are on the rise, demanding imminent action to revert this trend. Cardiovascular pathogenesis harbors a variety of molecular and cellular mechanisms among which dysregulated metabolism is of significant importance and may even proceed other mechanisms. The healthy heart metabolism primarily relies on fatty acids for the ultimate production of energy through oxidative phosphorylation in mitochondria. Other metabolites such as glucose, amino acids, and ketone bodies come next. Under pathological conditions, there is a shift in metabolic pathways and the preference of metabolites, termed metabolic remodeling or reprogramming. In this review, we aim to summarize cardiovascular metabolism and remodeling in different subsets of CVD to come up with a new paradigm for understanding and treatment of these diseases.
Graphical Abstract




Similar content being viewed by others
Abbreviations
- ACC2:
-
Acetyl-CoA carboxylase 2
- ACSL1:
-
Acyl-CoA synthetase long-chain family member 1
- ATGL:
-
Adipose triglyceride lipase
- AMP:
-
Adenosine monophosphate
- AMPK:
-
AMP-activated protein kinase
- ATP:
-
Adenosine triphosphate
- BCKD:
-
Branched-chain a-keto acid dehydrogenase
- BDH1:
-
β-Hydroxybutyrate dehydrogenase 1
- CPT1:
-
Carnitine palmitoyltransferase I
- DGAT1:
-
Diacylglycerol O-acyltransferase 1
- EAA:
-
Excitatory amino acid
- eNOS:
-
Endothelial nitric oxide synthase
- FAS:
-
Fatty acid synthase
- GLUT:
-
Glucose transporter
- LATs:
-
L-type amino acid transporters
- LPL:
-
Lipoprotein lipase
- MPC1:
-
Mitochondrial pyruvate carrier 1
- mTORC1:
-
Mammalian target of rapamycin complex 1
- NADH:
-
Nicotinamide adenine dinucleotide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- OXCT1:
-
3-Oxoacid CoA transferase 1
- PFK2:
-
Phosphofructokinase 2
- PFK1:
-
Phosphofructokinase 1
- PNPLA2:
-
Patatin-like phospholipase domain containing 2
- PON1:
-
Paraoxonase 1
- PON2:
-
Paraoxonase 2
- PPARA:
-
Peroxisome proliferator–activated receptor alpha
- PPARD:
-
Peroxisome proliferator–activated receptor delta
- PPARG:
-
Peroxisome proliferator–activated receptor gamma
- PPARGC1A:
-
PPARG coactivator 1 alpha
- PPARGC1B:
-
PPARG coactivator 1 beta
- PPM1K:
-
Protein phosphatase, Mg2+/Mn2+ dependent 1K
- ROS:
-
Reactive oxygen species
- SCOT:
-
Succinyl-CoA:3-oxoacid CoA transferase
- SLC2A1:
-
Solute carrier family 2 member 1
- SLC2A4:
-
Solute carrier family 2 member 4
- SLC16A1:
-
Solute carrier family 16 member 1
- SLC16A7:
-
Solute carrier family 16 member 7
- SLC27A1:
-
Solute carrier family 27 member 1
- TBC1D1:
-
TBC1 domain family member 1
- TBC1D4:
-
TBC1 domain family member 4
References
Joseph P, et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res. 2017;121:677–94.
Benjamin EJ, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492.
Barquera S, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–38.
Diez D, et al. The use of network analyses for elucidating mechanisms in cardiovascular disease. Mol BioSyst. 2010;6:289–304.
Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600.
Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res. 2018;123:107–28.
Wende AR, Brahma MK, McGinnis GR, Young ME. Metabolic origins of heart failure. Basic Trans Sci. 2017;2:297–310.
Ritterhoff J, Tian R. Metabolism in cardiomyopathy: every substrate matters. Cardiovasc Res. 2017;113:411–21.
Murashige D, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364–8.
Fukushima A, et al. Acetylation contributes to hypertrophy-caused maturational delay of cardiac energy metabolism. JCI Insight. 2018;3
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–513.
Karwi QG, Jörg AR, Lopaschuk GD. Allosteric, transcriptional and post-translational control of mitochondrial energy metabolism. Biochem J. 2019;476:1695–712.
Karwi QG, et al. Insulin directly stimulates mitochondrial glucose oxidation in the heart. Cardiovasc Diabetol. 2020;19:1–14.
Karwi QG, Uddin GM, Ho KL, Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med. 2018;5:68.
Ho KL, et al. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc Res. 2021;117:1178–87.
Karwi QG, Biswas D, Pulinilkunnil T, Lopaschuk GD. Myocardial ketones metabolism in heart failure. J Card Fail. 2020;26:998–1005.
Fillmore N, Wagg CS, Zhang L, Fukushima A, Lopaschuk GD. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol-Endocrinol Metabol. 2018;315:E1046–52.
Fahy E, et al. A comprehensive classification system for lipids1. J Lipid Res. 2005;46:839–61.
Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST. Neuronal lipid metabolism: multiple pathways driving functional outcomes in health and disease. Front Mol Neurosci. 2018;11:10.
Natesan V, Kim S-J. Lipid Metabolism, Disorders and Therapeutic Drugs–Review. Biomol Ther. 2021;29:596.
Nagy K, Tiuca I-D. Fatty acids. IntechOpen; 2017.
Leśniak W, et al. Cardiovascular risk management in type 2 diabetes of more than 10-year duration: results of Polish ARETAEUS2-Grupa Study. Cardiol J. 2015;22:150–9.
Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2017;8:1.
Uttaro AD. Biosynthesis of polyunsaturated fatty acids in lower eukaryotes. IUBMB Life. 2006;58:563–71.
Rosca MG, et al. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes. 2012;61:2074–83.
Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care: Clin Off Pract. 2013;40:195–211.
Mancini GJ, Hegele RA. Leiter, L. A. & Committee, D. C. C. P. G. E. Dyslipidemia. Can J Diabetes. 2018;42:S178–85.
Cohain AT, et al. An integrative multiomic network model links lipid metabolism to glucose regulation in coronary artery disease. Nat Commun. 2021;12:547.
Burdon KP, et al. Association of genes of lipid metabolism with measures of subclinical cardiovascular disease in the Diabetes Heart Study. J Med Genet. 2005;42:720–4.
Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805–12.
Marfella R, et al. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome [s]. J Lipid Res. 2009;50:2314–23.
McGavock JM, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–5.
Sharma S, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. The FASEB J. 2004;18:1692–700.
Labarthe F, Khairallah M, Bouchard B, Stanley WC, Des Rosiers C. Fatty acid oxidation and its impact on response of spontaneously hypertensive rat hearts to an adrenergic stress: benefits of a medium-chain fatty acid. Am J Physiol-Heart Circ Physiol. 2005;288:H1425–36.
Stowe KA, Burgess SC, Merritt M, Sherry AD, Malloy CR. Storage and oxidation of long-chain fatty acids in the C57/BL6 mouse heart as measured by NMR spectroscopy. FEBS Lett. 2006;580:4282–7.
Ballard FB, Danforth WH, Naegle S, Bing RJ. Myocardial metabolism of fatty acids. J Clin Invest. 1960;39:717–23.
Razani B, et al. Fatty acid synthase modulates homeostatic responses to myocardial stress. J Biol Chem. 2011;286:30949–61.
Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–9.
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58.
Perman JC, et al. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest. 2011;121:2625–40.
Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56.
Yagyu H, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–26.
Nöhammer C, et al. Myocardial dysfunction and male mortality in peroxisome proliferator-activated receptor alpha knockout mice overexpressing lipoprotein lipase in muscle. Lab Invest. 2003;83:259–69.
Chiu H-C, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–33.
Chiu H-C, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22.
Son N-H, et al. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–801.
Duncan JG, et al. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-α transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-α activators. Circulation. 2010;121:426–35.
Burkart EM, et al. Nuclear receptors PPARβ/δ and PPARα direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–9.
Cerk IK, Wechselberger L, Oberer M. Adipose triglyceride lipase regulation: an overview. Curr Protein and Pept Sci. 2018;19:221–33.
Haemmerle G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–7.
Haemmerle G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med. 2011;17:1076–85.
Cao Y, et al. Effects of Long-Chain Fatty Acyl-CoA Synthetase 1 on Diglyceride Synthesis and Arachidonic Acid Metabolism in Sheep Adipocytes. Int J Mol Sci. 21 https://doi.org/10.3390/ijms21062044(2020).
Ellis JM, et al. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol Cell Biol. 2011;31:1252–62.
Borradaile NM, et al. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–37.
Yan J, et al. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–28.
Son N-H, et al. PPARγ-induced cardiolipotoxicity in mice is ameliorated by PPARα deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120:3443–54.
Okere IC, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–23.
Chitraju C, Walther TC, Farese RV. The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes. J Lipid Res. 2019;60:1112–20.
Liu L, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–23.
Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555:1–13.
Tran DH, Wang ZV. Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc. 2019;8:e012673.
Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397–408.
Giugliano D, Ceriello A, Esposito K. Glucose metabolism and hyperglycemia. Am J Clin Nutr. 2008;87:217S–22S.
Rosano G, Fini M, Caminiti G, Barbaro G. Cardiac metabolism in myocardial ischemia. Curr Pharm Design. 2008;14:2551–62.
Chanda D, Luiken JJ, Glatz JF. Signaling pathways involved in cardiac energy metabolism. FEBS Lett. 2016;590:2364–74.
Nascimben L, et al. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–7.
Wang J, et al. Reduced cardiac fructose 2, 6 bisphosphate increases hypertrophy and decreases glycolysis following aortic constriction. PLoS One. 2013;8:e53951.
Leong H, Brownsey R, Kulpa J, Allard M. Glycolysis and pyruvate oxidation in cardiac hypertrophy—why so unbalanced? Comp Biochem Physiol A: Mol Integr Physiol. 2003;135:499–513.
Donthi RV, et al. Cardiac expression of kinase-deficient 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase inhibits glycolysis, promotes hypertrophy, impairs myocyte function, and reduces insulin sensitivity. J Biol Chem. 2004;279:48085–90.
Salt IP, Hardie DG. AMP-activated protein kinase: an ubiquitous signaling pathway with key roles in the cardiovascular system. Circ Res. 2017;120:1825–41.
Webster I, Friedrich SO, Lochner A, Huisamen B. AMP kinase activation and glut4 translocation in isolated cardiomyocytes. Cardiovasc J Afr. 2010;21:72–8.
Lee CT, Ussher JR, Mohammad A, Lam A, Lopaschuk GD. 5’-AMP-activated protein kinase increases glucose uptake independent of GLUT4 translocation in cardiac myocytes. Can J Physiol Pharmacol. 2014;92:307–14.
Cartee GD. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia. 2015;58:19–30. https://doi.org/10.1007/s00125-014-3395-5.
Habegger KM, Hoffman NJ, Ridenour CM, Brozinick JT, Elmendorf JS. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology. 2012;153:2130–41. https://doi.org/10.1210/en.2011-2099.
Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol-Endocrinol Metab. 2008;295:E29–37.
Hopkins TA, Dyck JR, Lopaschuk GD. AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart. Bioch Soc Trans. 2003;31:207–12. https://doi.org/10.1042/bst0310207.
Kantor PF, Robertson MA, Coe JY, Lopaschuk GD. Volume overload hypertrophy of the newborn heart slows the maturation of enzymes involved in the regulation of fatty acid metabolism. J Am College Cardiol. 1999;33:1724–34.
Mori J, et al. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ: Heart Fail. 2012;5:493–503.
Jenkins CM, Yang J, Sims HF, Gross RW. Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J Biol Chem. 2011;286:11937–50.
Izawa Y, et al. ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells. Exp Cell Res. 2005;308:291–9.
Zhang L, et al. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ: Heart Fail. 2013;6:1039–48.
Wang X, et al. Metabolic characterization of myocardial infarction using GC-MS-based tissue metabolomics. Int Heart J. 2017;58:441–6.
Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recoveryof intracellular pH and cardiac efficiency inpost-ischemic hearts by inhibiting glucose oxidation. J Am College Cardiol. 2002;39:718–25.
Gao Q, et al. Glycolysis and fatty acid β-oxidation, which one is the culprit of ischemic reperfusion injury. Int J Clin Exp Med. 2018;11:59–68.
Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation—a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta (BBA)-Mol Cell Res. 2011;1813:1333–50.
Nissler K, Petermann H, Wenz I, Brox D. Fructose 2, 6-bisphosphate metabolism in Ehrlich ascites tumour cells. J Cancer Res Clin Oncol. 1995;121:739–45.
Opie LH. Myocardial ischemia—metabolic pathways and implications of increased glycolysis. Cardiovasc Drugs Ther. 1990;4:777–90.
Chaudhry R, Varacallo M. Biochemistry, glycolysis. StatPearls Publishing; 2018.
Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–5.
Fillmore N, Mori J, Lopaschuk G. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014;171:2080–90.
Beltran C, et al. Enhancing glycolysis protects against ischemia-reperfusion injury by reducing ROS production. Metabolites. 2020;10:132.
Paolisso G, et al. Total-body and myocardial substrate oxidation in congestive heart failure. Metabolism. 1994;43:174–9.
Lommi J, Kupari M, Yki-Järvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol. 1998;81:45–50.
Lionetti V, Stanley WC, Recchia FA. Modulating fatty acid oxidation in heart failure. Cardiovasc Res. 2011;90:202–9.
Fillmore N, et al. Uncoupling of glycolysis from glucose oxidation accompanies the development of heart failure with preserved ejection fraction. Mol Med. 2018;24:1–12.
Diakos NA, et al. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: implications for cardiac reloading and conditioning. JACC: Basic Trans Sci. 2016;1:432–44.
Huang Y, Zhou M, Sun H, Wang Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovasc Res. 2011;90:220–3.
Karwi QG, Lopaschuk GD. Branched-chain amino acid metabolism in the failing heart. Cardiovasc Drugs Ther. 2022:1–8.
Kandasamy P, Gyimesi G, Kanai Y, Hediger MA. Amino acid transporters revisited: new views in health and disease. Trends Biochem Sci. 2018;43:752–89.
Carpentier AC. Branched-chain amino acid catabolism by brown adipose tissue. Endocrinology. 2020;161:bqaa060.
Zhang B, et al. Leucine supplementation in a chronically protein-restricted diet enhances muscle weight and postprandial protein synthesis of skeletal muscle by promoting the mTOR pathway in adult rats. Engineering. 2017;3:760–5.
Romano S, et al. Cardiomyopathies in propionic aciduria are reversible after liver transplantation. J Pediatr. 2010;156:128–34.
De Bie I, Nizard SDP, Mitchell GA. Fetal dilated cardiomyopathy: an unsuspected presentation of methylmalonic aciduria and hyperhomocystinuria, cblC type. Prenat diagn. 2009;29:266–70.
Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun. 2004;313:391–6.
Lu G, et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119:1678–87.
Manoli I, Venditti C. Disorders of branched chain amino acid metabolism. Trans Sci Rare Dis. 2016;1:91–110.
Lu G, et al. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–96.
Katta A, et al. Impaired overload-induced hypertrophy is associated with diminished mTOR signaling in insulin-resistant skeletal muscle of the obese Zucker rat. Am J Physiol-Regul Integr Comp Physiol. 2010;299:R1666–75.
Li Y, et al. AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch Biochem Biophys. 2014;558:79–86.
D'Antona G, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362–72.
de Keyzer Y, et al. Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr Res. 2009;66:91–5.
Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141:1800–12.
Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/Metab Res Rev. 1999;15:412–26.
Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–84.
van Hasselt PM, et al. Monocarboxylate transporter 1 deficiency and ketone utilization. New Engl J Med. 2014;371:1900–7.
Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60:143–87.
Kadir AA, Clarke K, Evans RD. Cardiac ketone body metabolism. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2020;1866:165739.
Russell R, Taegtmeyer H. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest. 1991;87:384–90.
Russell R 3rd, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol-Heart Circ Physiol. 1991;261:H1756–62.
Takahara S, Soni S, Maayah ZH, Ferdaoussi M, Dyck JR. Ketone therapy for heart failure: current evidence for clinical use. Cardiovasc Res. 2022;118:977–87.
Schugar RC, et al. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. 2014;3:754–69.
Aubert G, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705.
Horton JL, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4
De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33:860–71.
Keon CA, et al. Substrate dependence of the mitochondrial energy status in the isolated working rat heart. Biochem Soc Trans. 1995;307S.
Bassenge E, et al. Effect of ketone bodies on cardiac metabolism. Am J Physiol-Legacy Content. 1965;208:162–8.
Stanley WC, Meadows SR, Kivilo KM, Roth BA, Lopaschuk GD. β-Hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Am J Physiol-Heart Circ Physiol. 2003
Bedi KC Jr, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–16.
Uchihashi M, et al. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload–induced heart failure. Circ: Heart Fail. 2017;10:e004417.
Lopaschuk GD, Wall SR, Olley PM, Davies NJ. Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circ Res. 1988;63:1036–43.
Turcani M, Rupp H. Etomoxir improves left ventricular performance of pressure-overloaded rat heart. Circulation. 1997;96:3681–6.
Rupp H, Vetter R. Sarcoplasmic reticulum function and carnitine palmitoyltransferase-1 inhibition during progression of heart failure. Br J Pharmacol. 2000;131:1748–56.
Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci. 2000;99:27–35.
Lee L, et al. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–8.
Fragasso G, et al. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–8.
Tuunanen H, et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008;118:1250–8.
Malmberg K, et al. Effects of insulin treatment on cause-specific one-year mortality and morbidity in diabetic patients with acute myocardial infarction. Eur Heart J. 1996;17:1337–44.
Díaz R, et al. Metabolic modulation of acute myocardial infarction. The ECLA (Estudios Cardiológicos Latinoamérica) Collaborative Group. Circulation. 1998;98:2227–34.
Jonassen AK, Aasum E, Riemersma RA, Mjøs OD, Larsen TS. Glucose-insulin-potassium reduces infarct size when administered during reperfusion. Cardiovasc. Drugs Ther. 2000;14:615–23.
van der Horst IC, et al. Glucose-insulin-potassium infusion inpatients treated with primary angioplasty for acute myocardial infarction: the glucose-insulin-potassium study: a randomized trial. J Am College Cardiol. 2003;42:784–91.
Ceremużyński L, et al. Low-dose glucose-insulin-potassium is ineffective in acute myocardial infarction: results of a randomized multicenter Pol-GIK trial. Cardiovasc Drugs Ther. 1999;13:191–200.
van der Horst IC, et al. Glucose-insulin-potassium and reperfusion in acute myocardial infarction: rationale and design of the Glucose-Insulin-Potassium Study-2 (GIPS-2). Am Heart J. 2005;149:585–91.
Funding
This work was supported by the National Natural Science Foundation of China (82070398, 81922008), Key Basic Research Projects of Basic Strengthening Plan (2022-JCJQ-ZD-095-00), and the Top Young Talents Special Support Program in Shaanxi Province (2020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Yihua Bei oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Shen, M., Shu, X. et al. Cardiac Metabolism, Reprogramming, and Diseases. J. of Cardiovasc. Trans. Res. 17, 71–84 (2024). https://doi.org/10.1007/s12265-023-10432-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10432-3