Abstract
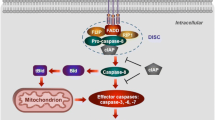
Programmed cardiac myocyte death via the intrinsic, or mitochondrial, pathway is a mechanism of pathological ventricular remodeling after myocardial infarction and during chronic pressure overload hypertrophy. Transcriptional upregulation of the closely related proapoptotic Bcl2 family members BNip3 in ischemic myocardium and Nix in hypertrophied myocardium suggested a molecular mechanism by which programmed cell death can be initiated by cardiac stress and lead to dilated cardiomyopathy. Studies using transgenic and gene knockout mice subsequently demonstrated that expression of BNip3 and Nix is both sufficient for cardiomyopathy development and necessary for cardiac remodeling after reversible coronary occlusion and transverse aortic banding, respectively. Here, these data are reviewed in the context of recent findings showing that Nix not only stimulates cardiomyocyte apoptosis but also induces mitochondrial autophagy (mitophagy) and indirectly activates the mitochondrial permeability transition pore, causing cell necrosis. New findings are presented suggesting that Nix and BNip3 have an essential function, “mitochondrial pruning,” that restrains mitochondrial proliferation in cardiomyocytes and without which an age-dependent mitochondrial cardiomyopathy develops.






Similar content being viewed by others
References
Diwan, A., & Dorn, G. W., II. (2007). Decompensation of cardiac hypertrophy: Cellular mechanisms and novel therapeutic targets. Physiology (Bethesda), 22, 56–64.
Berry, J. J., Hoffman, J. M., Steenbergen, C., Baker, J. A., Floyd, C., Van Trigt, P., et al. (1993). Human pathologic correlation with PET in ischemic and nonischemic cardiomyopathy. Journal of Nuclear Medicine, 34, 39–47.
Olivetti, G., Abbi, R., Quaini, F., Kajstura, J., Cheng, W., Nitahara, J. A., et al. (1997). Apoptosis in the failing human heart. Journal of Nuclear Medicine, 336, 1131–1141.
Francis, G. S. (1998). Changing the remodeling process in heart failure: Basic mechanisms and laboratory results. Current Opinion in Cardiology, 13, 156–161.
Olivetti, G., Capasso, J. M., Sonnenblick, E. H., & Anversa, P. (1990). Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circulation Research, 67, 23–34.
Rubart, M., & Field, L. J. (2006). Cardiac regeneration: Repopulating the heart. Annual Review of Physiology, 68, 29–49.
Hayakawa, Y., Chandra, M., Miao, W., Shirani, J., Brown, J. H., Dorn, G. W., II, et al. (2003). Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation, 108, 3036–3041.
Foo, R. S., Mani, K., & Kitsis, R. N. (2005). Death begets failure in the heart. Journal of Clinical Investigation, 115, 565–571.
Dorn, G. W., II. (2009). Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovascular Research, 81, 465–473.
Youle, R. J., & Strasser, A. (2008). The BCL-2 protein family: Opposing activities that mediate cell death. Nature Reviews. Molecular Cell Biology, 9, 47–59.
Chen, Z., Chua, C. C., Ho, Y. S., Hamdy, R. C., & Chua, B. H. (2001). Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. American Journal of Physiology. Heart and Circulatory Physiology, 280, H2313–H2320.
Condorelli, G., Morisco, C., Stassi, G., Notte, A., Farina, F., Sgaramella, G., et al. (1999). Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation, 99, 3071–3078.
Yussman, M. G., Toyokawa, T., Odley, A., Lynch, R. A., Wu, G., Colbert, M. C., et al. (2002). Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nature Medicine, 8, 725–730.
Regula, K. M., Ens, K., & Kirshenbaum, L. A. (2002). Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circulation Research, 91, 226–231.
Galvez, A. S., Brunskill, E. W., Marreez, Y., Benner, B. J., Regula, K. M., Kirschenbaum, L. A., et al. (2006). Distinct pathways regulate proapoptotic Nix and BNip3 in cardiac stress. Journal of Biological Chemistry, 281, 1442–1448.
Yurkova, N., Shaw, J., Blackie, K., Weidman, D., Jayas, R., Flynn, B., et al. (2008). The cell cycle factor E2F-1 activates Bnip3 and the intrinsic death pathway in ventricular myocytes. Circulation Research, 102, 472–479.
Bruick, R. K. (2000). Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proceedings of the National Academy of Sciences of the United States of America, 97, 9082–9087.
Sowter, H. M., Ratcliffe, P. J., Watson, P., Greenberg, A. H., & Harris, A. L. (2001). HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Research, 61, 6669–6673.
Birse-Archbold, J. L., Kerr, L. E., Jones, P. A., McCulloch, J., & Sharkey, J. (2005). Differential profile of Nix upregulation and traslocation during hypxia/ischaemia in vivo versus in vitro. Journal of Cerebral Blood Flow and Metabolism, 25, 1356–1365.
Syed, F., Odley, A., Hahn, H. S., Brunskill, E. W., Lynch, R. A., Marreez, Y., et al. (2004). Physiological growth synergizes with pathological genes in experimental cardiomyopathy. Circulation Research, 95, 1200–1206.
Dorn, G. W., II. (2005). Physiologic growth and pathologic genes in cardiac development and cardiomyopathy. Trends in Cardiovascular Medicine, 15, 185–189.
Diwan, A., Krenz, M., Syed, F. M., Wansapura, J., Ren, X., Koesters, A. G., et al. (2007). Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. Journal of Clinical Investigation, 117, 2825–2833.
Kubasiak, L. A., Hernandez, O. M., Bishopric, N. H., & Webster, K. A. (2002). Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proceedings of the National Academy of Sciences of the United States of America, 99, 12825–12830.
Kubli, D. A., Quinsay, M. N., Huang, C., Lee, Y., & Gustafsson, A. B. (2008). Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. American Journal of Physiology. Heart and Circulatory Physiology, 295, H2025–H2031.
Schmidt-Kastner, R., Aguirre-Chen, C., Kietzmann, T., Saul, I., Busto, R., & Ginsberg, M. D. (2004). Nuclear localization of the hypoxia-regulated pro-apoptotic protein BNIP3 after global brain ischemia in the rat hippocampus. Brain Research, 1001, 133–142.
Burton, T. R., Henson, E. S., Baijal, P., Eisenstat, D. D., & Gibson, S. B. (2005). The pro-cell death Bcl-2 family member, BNIP3, is localized to the nucleus of human glial cells: Implications for glioblastoma multiforme tumor cell survivial under hypoxia. International Journal of Cancer, 118, 1660–1669.
Diwan, A., Koesters, A. G., Odley, A. M., Pushkaran, S., Baines, C. P., Spike, B. T., et al. (2007). Unrestrained erythroblast development in Nix−/− mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proceedings of the National Academy of Sciences of the United States of America, 104, 6794–6799.
Schweers, R. L., Zhang, J., Randall, M. S., Loyd, M. R., Li, W., Dorsey, F. C., et al. (2007). NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proceedings of the National Academy of Sciences of the United States of America, 104, 19500–19505.
Sandoval, H., Thiagarajan, P., Dasgupta, S. K., Schumacher, A., Prchal, J. T., Chen, M., et al. (2008). Essential role for Nix in autophagic maturation of erythroid cells. Nature, 454, 232–235.
Diwan, A., Wansapura, J., Syed, F. M., Matkovich, S. J., Lorenz, J. N., & Dorn, G. W., II. (2008). Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation, 117, 396–404.
Diwan, A., Matkovich, S. J., Yuan, Q., Zhao, W., Yatani, A., Brown, J. H., et al. (2009). Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. Journal of Clinical Investigation, 119, 203–212.
Foyouzi-Youssefi, R., Arnaudeau, S., Borner, C., Kelley, W. L., Tschopp, J., Lew, D. P., et al. (2000). Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America, 97, 5723–5728.
Nutt, L. K., Pataer, A., Pahler, J., Fang, B., Roth, J., McConkey, D. J., et al. (2002). Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. Journal of Biological Chemistry, 277, 9219–9225.
Scorrano, L., Oakes, S. A., Opferman, J. T., Cheng, E. H., Sorcinelli, M. D., Pozzan, T., et al. (2003). BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science, 300, 135–139.
Rizzuto, R., & Pozzan, T. (2006). Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiological Reviews, 86, 369–408.
Henriquez, M., Armisen, R., Stutzin, A., & Quest, A. F. G. (2008). Cell death by necrosis, a regulated way to go. Current Molecular Medicine, 8, 187–206.
Nakayama, H., Chen, X., Baines, C. P., Klevitsky, R., Zhang, X., Zhang, H., et al. (2007). Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. Journal of Clinical Investigation, 117, 2431–2444.
Dorn, G. W., II, & Kirshenbaum, L. A. (2008). Cardiac reanimation: Targeting cardiomyocyte death by BNIP3 and NIX/BNIP3L. Oncogene, 27(Suppl 1), S158–S167.
Zhang, J., & Ney, P. A. (2009). Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death and Differentiation, 16, 939–946.
Lehman, J. J., Barger, P. M., Kovacs, A., Saffitz, J. E., Medeiros, D., & Kelly, D. P. (2000). PPARg coactivator-1 (PGC-1) promotes cardiac mitochondrial biogenesis. Journal of Clinical Investigation, 106, 847–856.
Russell, L. K., Mansfield, C. M., Lehman, J. J., Kovacs, A., Courtois, M., Saffitz, J. E., et al. (2004). Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor g coactivator-1a promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circulation Research, 94, 525–533.
Zhang, J., & Ney, P. A. (2008). NIX induces mitochondrial autophagy in reticulocytes. Autophagy, 4, 354–356.
Schwarten, M., Mohrluder, J., Ma, P., Stoldt, M., Thielmann, Y., Stangler, T., et al. (2009). Nix directly binds to GABARAP: A possible crosstalk between apoptosis and autophagy. Autophagy, 5, 690–698.
Novak, I., Kirkin, V., McEwan, D. G., Zhang, J., Wild, P., Rozenknop, A., et al. (2010). Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep, 11, 45–51.
Goffart, S., Kleist-Retzow, J. C., & Wiesner, R. J. (2004). Regulation of mitochondrial proliferation in the heart: Power-plant failure contributes to cardiac failure in hypertrophy. Cardiovascular Research, 64, 198–207.
Acknowledgments
Supported by NIH HL059888. The author would like to express his deep appreciation to the many members of his laboratory who contributed in various ways to the Nix story over the past decade, especially to those who were tolerant of the idea that we should maintain some mice for long periods of time just to see what might develop.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorn, G.W. Mitochondrial Pruning by Nix and BNip3: An Essential Function for Cardiac-Expressed Death Factors. J. of Cardiovasc. Trans. Res. 3, 374–383 (2010). https://doi.org/10.1007/s12265-010-9174-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-010-9174-x