Abstract
A large inter-individual variability in the plasma triglyceride (TG) response to fish oil consumption has been observed. The objective was to investigate the gene–diet interaction effects between single-nucleotide polymorphisms (SNPs) within glucokinase (GCK) gene and dietary carbohydrate intakes (CHO) on the plasma TG response to a fish oil supplementation. Two hundred and eight participants were recruited in the greater Quebec City area. The participants completed a 6-week fish oil supplementation (5 g fish oil/day: 1.9–2.2 g EPA and 1.1 g DHA). Thirteen SNPs within GCK gene were genotyped using TAQMAN methodology. A gene–diet interaction effect on the plasma TG response was observed with rs741038 and CHO adjusted for age, sex and BMI (p = 0.008). In order to compare the plasma TG response between genotypes according to CHO, participants were divided according to median CHO. Homozygotes of the minor C allele of rs741038 with high CHO >48.59 % had a greater decrease in their plasma TG concentrations following the intake of fish oil (p < 0.05) than C/C homozygotes with low CHO and also than the other genotypes either with high or low CHO. The plasma TG response to a fish oil supplementation may be modulated by gene–diet interaction effects involving GCK gene and CHO.
Similar content being viewed by others
Introduction
Glucokinase (GCK) gene encodes for GCK or hexokinase type IV which phosphorylates glucose to glucose-6-phosphate (Massa et al. 2011). GCK gene is expressed mainly in the liver and in pancreatic beta cells but also in neurons, the pituitary and the entero-endocrine K and L cells (Massa et al. 2011). GCK has a glucose-sensor role and regulates glucose disposal toward glycogenesis and TG synthesis (Iynedjian 2009). It is partly regulated by GCKR which forms an inhibitory complex with GCK at low glucose concentrations because of its binding with fructose-6-phosphate (Choi et al. 2013). At high glucose concentrations such as after a meal high in carbohydrates (CHO), fructose-1-phosphate binds with GCKR and releases GCK which enhances glucose metabolism leading to a greater glycolytic flux (Girard et al. 1997b; Iynedjian 2009). A few single-nucleotide polymorphisms (SNPs) within GCK have been reported to have an impact on type 2 diabetes risk, glycemia, beta cell function and plasma TG concentrations (Hu et al. 2010; Sotos-Prieto et al. 2013; Wang et al. 2013; Fu et al. 2013; Asselbergs et al. 2012).
The hypotriglyceridemic impact of marine omega-3 polyunsaturated fatty acids (n-3 PUFA) at doses around 3 g or more is well known (De 2011). This effect is even observed among individuals with plasma TG concentrations within normal values (<1.7 mmol/L) and at lower marine n-3 PUFA doses (<2 g) (Caslake et al. 2008). However, an important inter-individual variability in the plasma TG response to marine n-3 PUFA has been reported (Caslake et al. 2008; Cormier et al. 2012; Minihane et al. 2000). Approximately 30 % of the individuals do not lower their plasma TG concentrations following the intake of marine n-3 PUFA (Cormier et al. 2012; Madden et al. 2011). The reduction in de novo lipogenesis pathway is one of the mechanisms which may explain the hypotriglyceridemic effect of n-3 PUFA (Harris and Bulchandani 2006). Among healthy individuals, the proportion of free fatty acids from de novo lipogenesis contributing to the free fatty acids pool that are incorporated into the VLDL-TG assembly is relatively small (~5 %) (Vedala et al. 2006). However, among hypertriglyceridemic obese individuals with or without type 2 diabetes, the contribution of de novo lipogenesis increases to around 13–14 % (Vedala et al. 2006). De novo lipogenesis is modulated by high dietary carbohydrate (CHO) intakes which increase the activity of GCK, the production of acetyl-CoA and malonyl-CoA, therefore stimulating de novo lipogenesis (Girard et al. 1997a; Iynedjian 2009). Thus, we hypothesized that SNPs within GCK gene could have an impact on the plasma TG response to a fish oil supplementation and these impacts could be modulated by gene–diet interaction effects with dietary CHO.
Methods
Participants
A total of 254 unrelated participants were recruited to participate in this clinical trial from the greater Quebec City metropolitan area between September 2009 and December 2011 through advertisements in local newspapers as well as by electronic messages sent to university students/employees (Bouchard-Mercier et al. 2013). Briefly, to be eligible, participants had to be non-smokers and free of any thyroid or metabolic disorders requiring treatment such as diabetes, hypertension, severe dyslipidemia and coronary heart disease. A total of 210 participants completed the n-3 PUFA supplementation period. However, plasma TG concentrations were available for 208 participants, and thus, the analyses were conducted on 208 participants. No differences in the descriptive characteristics were observed between the participants included in these analyses and the participants who did not complete the study or for which plasma TG concentrations values were missing. The experimental protocol was approved by the ethics committees of Laval University Hospital Research Center and Laval University. This clinical trial was registered at clinicaltrials.gov (NCT01343342). Informed written consent was obtained from all the study participants.
Study design and diets
The study design and diets have been described previously (Bouchard-Mercier et al. 2013). Briefly, participants followed a run-in period of 2 weeks. Individual dietary instructions were given by a trained dietitian to achieve the recommendations from Canada’s Food Guide in order to standardize dietary intakes. Some specifications were given regarding the n-3 PUFA dietary intake: not exceed two fish or seafood servings per week, prefer white flesh fishes instead of fatty fishes (examples were given) and avoid enriched n-3 PUFA dietary products such as some milks, juices, breads and eggs. Subjects were also asked to limit their alcohol consumption during the protocol; two regular drinks per week were allowed. In addition, subjects were not allowed to take n-3 PUFA supplements (such as flaxseed), vitamins or natural health products during the protocol.
After the 2-week run-in period, each participant received a bottle containing n-3 PUFA capsules for the next 6 weeks. They were instructed to take five capsules (1 g of fish oil/capsule) per day (Ocean Nutrition, Nova Scotia, Canada), providing a total of 5 g of fish oil (1.9–2.2 g EPA and 1.1 g DHA) per day.
Participants completed two 3-d food records, before and after the n-3 PUFA supplementation period. Thus, the first food record reflected dietary intakes of the week prior to the beginning of the supplementation period and for the second food record the week prior to the end of the supplementation period. All foods and beverages consumed on 2 representative weekdays and 1 weekend day were weighed or estimated and recorded in food diaries. Dietary intake data were analyzed using Nutrition Data System for Research software version 2011 developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN.
Biochemical parameters
The morning after a 12-h overnight fast and 48-h alcohol abstinence, blood samples were collected from an antecubital vein into vacutainer tubes containing EDTA. Plasma was separated by centrifugation (2,500×g for 10 min at 4 °C), and samples were aliquoted and frozen for subsequent analyses. Plasma total cholesterol (total-C) and plasma TG concentrations were measured using enzymatic assays (Burstein and Samaille 1960; McNamara and Schaefer 1987). Infranatant (d > 1.006 g/ml) with heparin-manganese chloride was used to precipitate VLDL and LDL and then determine HDL-cholesterol concentrations (HDL-C) (Albers et al. 1978). The equation of Friedewald was used to estimate LDL-cholesterol concentrations (LDL-C) (Friedewald et al. 1972). Non-HDL-C was calculated by subtracting HDL-C from total-C.
SNPs selection and genotyping
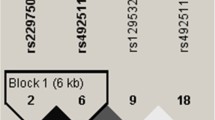
As described previously (Bouchard-Mercier et al. 2013), SNPs were selected with the International HapMap Project SNP database (HapMap Data Rel 28 Phase II + III, August 10, on National Center for Biotechnology Information (NCBI) B36 assembly, dbSNP b126). The chromosomal region selected was chr7:44149895-44198063, which included 2,500 bp upstream and 500 bp downstream of GCK gene. Tag SNPs (tSNPs) were determined with the tagger procedure in HaploView software version 4.2 with minor allele frequency (MAF) of >0.05 and pairwise tagging R 2 ≥ 0.80. For each gene, a minimum of 85 % of the most common SNPs had to be captured by tSNPs. Afterward, as shown in Fig. 1, linkage disequilibrium (LD) plot was generated with Haploview software version 4.2. All tSNPs were genotyped within INAF laboratories with the TAQMAN methodology (Livak 1999), as described previously (Cormier et al. 2012). Briefly, genotypes were determined using ABI Prism SDS version 2.0.5 (Applied Biosystem, Foster City, CA, USA). All SNPs were successfully genotyped with a call rate of 100 % (n = 208).
Statistical analyses
Hardy–Weinberg equilibrium was tested with the ALLELE procedure of SAS version 9.3 using Fisher’s exact test (p < 0.01). When the genotype frequency for homozygous individuals of the minor allele was <5 %, carriers (heterozygotes and homozygotes) of the minor allele were grouped.
Variables non-normally distributed were logarithmically transformed (BMI, plasma TG and insulin). Differences were assessed using analyses of variance (ANOVAs) with the GLM procedure in SAS and the type 3 sum of squares for unbalanced study design. To examine the impact of gene–diet interaction effects on the plasma TG response, the interaction term (SNP × CHO) was added into the ANOVA model. The plasma TG response was calculated as followed: ((post-supplementation plasma TG − pre-supplementation plasma TG)/pre-supplementation plasma TG × 100). The model was adjusted for the effects of age, sex and BMI. To take into account the effects of multiple testing, the simpleM procedure described by Gao et al. (2008) was used. The number of effective independent test determined by the procedure of Gao et al. (2008) was eight, and only one environmental factor (CHO) was studied. Thus, p values lower than 6.25 × 10−3 were considered significant (p = 0.05/(8 × 1)). All statistical analyses were performed using SAS statistical software version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Descriptive characteristics of the study population and tSNPs
As previously observed (Thifault et al. 2013), the baseline, pre- and post-supplementation descriptive characteristics including dietary intakes of the study participants are presented in Table 1. Briefly, BMI, fasting glucose and HDL-C slightly increased (p = 0.005, p = 0.0003 and p = 0.005, respectively), whereas plasma TG decreased (p < 0.0001) following the fish oil supplementation. For dietary intakes, total daily energy intakes (including fish oil supplements) and CHO (expressed as percentage of energy intakes) decreased (p = 0.006 and p = 0.0009, respectively) and total fat intakes (expressed as percentage of energy intakes) increased (p = <0.0001) following the fish oil supplementation.
The selected SNPs are presented in Online Resource 1. All SNPs were in Hardy–Weinberg equilibrium (p < 0.01). Figure 1 presents the LD plot. Briefly, thirteen tSNPs covered 86 % of the known genetic variability within GCK gene (including 2,500 bp upstream and 500 bp downstream GCK gene).
The impact of CHO and tSNPs on pre-supplementation plasma TG concentrations
No gene–diet interaction effects on pre-supplementation plasma TG concentrations were observed (data not shown). Pre-supplementation CHO intake was positively associated with pre-supplementation plasma TG concentrations (r = 0.19, p = 0.007). Pre-supplementation plasma TG concentrations according to genotype are presented in Table 2. Briefly, no differences in plasma TG concentrations were observed according to genotypes. A trend (p = 0.07) was observed for rs3757838, for which individuals carrying the A allele had lower plasma TG concentrations than T/T homozygotes.
The impact of tSNPs, CHO and gene–diet interaction effects on the plasma TG response
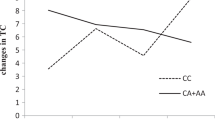
A trend for an inverse relationship between the plasma TG response and post-supplementation CHO was observed (r = −0.14, p = 0.05). This association was also observed between the plasma TG response and pre-supplementation CHO (r = −0.19, p = 0.008). As shown in Table 3, gene–diet interaction effects on the plasma TG response were observed with rs741038 and post-supplementation CHO expressed as percentage of energy consumption and adjusted for age, sex and BMI (p = 0.008) and was significant after correction for multiple testing only when pre-supplementation plasma TG concentrations were included into the model as a confounding variable (p = 0.001). A trend for a gene–diet interaction effect was also observed for rs1990458 (p = 0.07) when age, sex and BMI were included in the model. None of the other SNPs were associated with the plasma TG response either alone or in interaction with CHO. To further explore the impact of rs741038 and CHO, participants were divided on the basis of CHO using the median value and genotype. As shown in Fig. 2, homozygotes for the minor C allele of rs741038 with high CHO >48.59 % had a greater decrease in plasma TG concentrations following the intake of fish oil than C/C homozygotes with low CHO intake (p = 0.002), than C/T heterozygotes with either low or high CHO (p = 0.03 and p = 0.005, respectively) and than T/T homozygotes with either low or high CHO intakes (p = 0.02 and p = 0.04, respectively).
The plasma TG response (%) according to median CHO and rs741038 genotype. The plasma TG response following fish oil supplementation (mean ± SE). Means with different letters are significantly different (assessed by an ANOVA). CHO are separated according to the median value: ≤48.59 (low in light color) and >48.59 % (high in dark color). Number of participants: low CHO and C/C genotype (n = 13), high CHO and C/C genotype (n = 10), low CHO and C/T genotype (n = 44), high CHO and C/T genotype (n = 49), low CHO and T/T genotype (n = 47), high CHO and T/T genotype (n = 45)
Discussion
In this study, gene–diet interaction effects on the plasma TG response to fish oil were observed between GCK gene and CHO. The intake of CHO modified the plasma TG response to fish oil depending on the genotype of rs741038 within GCK gene. For C/C homozygotes, low CHO intakes were associated with an increase in plasma TG concentrations, whereas when CHO intakes were high, they had the greatest decrease in their plasma TG concentrations following the fish oil supplementation of all genotype groups. The SNP rs741038 is an intronic SNP with no potential regulatory impact and is not in LD with rs1799884 or rs2070971 which have been associated previously with plasma TG levels, fasting glucose and/or type 2 diabetes risk (Asselbergs et al. 2012; Holmkvist et al. 2008; Sotos-Prieto et al. 2013; Webster et al. 2009; Hu et al. 2010; Wang et al. 2013). However, it is possible that rs741038 is in LD with other unknown functional SNPs. Moreover, the association between rs1799884 and plasma TG concentrations was not confirmed by our results (Sotos-Prieto et al. 2013). Hishida et al. (2012) have investigated the impact of gene–diet interaction effect on the risk of dyslipidemia defined as elevated plasma TG and/or low HDL-C concentrations and did not observe interaction effects with GCK gene rs1799884 and the intakes of energy, fat, CHO or alcohol. A recent meta-analysis has shown that the association between rs1799884 and type 2 diabetes varies according to ethnic populations and could be more important among Caucasians than among Asians (Fu et al. 2013). In a large-scale gene-centric meta-analysis, the intronic SNP rs2070971 within GCK gene was associated with plasma TG concentrations (Asselbergs et al. 2012).
In the liver, GCK gene expression is regulated by the presence of insulin (Iynedjian et al. 1988; Iynedjian 2009). This mechanism involves the long-term regulation of GCK gene transcription and translation (Moore et al. 2012). The induction of GCK gene expression by insulin may be modulated by dietary components such as dietary PUFA which decrease GCK gene expression induction (Jump et al. 1994). Moreover, the long-term consumption of a diet high in fat and in fructose among dogs has shown to also decrease GCK protein and its activity in the liver as well as glycogen storage, but not mRNA abundance (Coate et al. 2010, 2011; Moore et al. 2012). It has been observed that GCK mRNA abundance is positively correlated with the liver TG content (Peter et al. 2011). An increased GCK gene expression was also positively associated with expression levels of lipogenic enzymes and de novo lipogenesis index (Peter et al. 2011). It has been observed that compared to a high-fat/low-CHO diet, a low-fat/high-CHO diet led to increases in de novo lipogenesis which was correlated with the increase in plasma TG concentrations among both normoinsulinemic and hyperinsulinemic individuals (Schwarz et al. 2003). In this study, there was a positive correlation between pre-supplementation CHO and plasma TG concentrations. However, following the intake of fish oil, an inverse relationship between CHO (both pre- and post-supplementation) and the plasma TG response was observed. Thus, it is possible that higher pre-supplementation CHO leads to higher GCK activity and, therefore, enhances the potential of fish oil to decrease its activity and affect plasma TG concentrations.
The variability observed in the plasma TG response to fish oil could be attributable to factors such as poor compliance. However, compliance rates were high (94 %) (Bouchard-Mercier et al. 2013), and thus, poor compliance could not explain the variability observed. Sex is another factor that may modulate the plasma TG response to fish oil (Caslake et al. 2008); however, no sex effect (or statistical interaction with sex) was observed in this cohort (Thifault et al. 2013). In our previous work, differences between responders and non-responders to fish oil intake according to the plasma TG response in the modulation of the expression levels of many genes involved in glycerophospholipid, sphingolipid, arachidonic acid and linoleic acid metabolisms were observed (Rudkowska et al. 2012). It is possible that pre-supplementation gene expression levels, depending on the dietary intakes or other environmental parameters, may affect the plasma TG response to fish oil.
Conclusion
A gene–diet interaction effect between GCK gene and CHO on the plasma TG response following fish oil intake among overweight individuals has been observed. For homozygotes of the minor allele, higher CHO intakes led to important decreases in plasma TG concentrations following the fish oil supplementation. However, when their intake of CHO was lower, they slightly increased their plasma TG concentrations. Still, for homozygotes of the minor allele with lower CHO intakes, plasma TG concentrations were within normal values. Thus, they are unlikely to be at increased cardiovascular disease risk following fish oil supplementation. These results need to be replicated among other populations. In the future, considering dietary carbohydrate intakes and the genetic background before recommending fish oil to reduce plasma TG concentrations may be of importance to identify subgroups of the population that may not respond as predicted. These results reinforce the importance of considering inter-individual variability in the response to fish oil supplementation. It will also be necessary to achieve a global understanding of the impacts of several gene–diet interaction effects on the plasma TG response to fish oil before taking this knowledge into clinical practice.
References
Albers JJ, Warnick GR, Wiebe D, King P, Steiner P, Smith L, Breckenridge C, Chow A, Kuba K, Weidman S, Arnett H, Wood P, Shlagenhaft A (1978) Multi-laboratory comparison of three heparin-Mn2+ precipitation procedures for estimating cholesterol in high-density lipoprotein. Clin Chem 24:853–856
Asselbergs FW, Guo Y, van Iperen EP, Sivapalaratnam S, Tragante V, Lanktree MB et al (2012) Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. Am J Hum Genet 91:823–838
Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl MC (2013) Polymorphisms, de novo lipogenesis and plasma triglyceride response following fish oil supplementation. J Lipid Res 54:2866–2873
Burstein M, Samaille J (1960) On a rapid determination of the cholesterol bound to the serum alpha- and beta-lipoproteins. Clin Chim Acta 5:609
Caslake MJ, Miles EA, Kofler BM, Lietz G, Curtis P, Armah CK, Kimber AC, Grew JP, Farrell L et al (2008) Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. Am J Clin Nutr 88:618–629
Choi JM, Seo MH, Kyeong HH, Kim E, Kim HS (2013) Molecular basis for the role of glucokinase regulatory protein as the allosteric switch for glucokinase. Proc Natl Acad Sci USA 110:10171–10176
Coate KC, Scott M, Farmer B, Moore MC, Smith M, Roop J, Neal DW, Williams P, Cherrington AD (2010) Chronic consumption of a high-fat/high-fructose diet renders the liver incapable of net hepatic glucose uptake. Am J Physiol Endocrinol Metab 299:E887–E898
Coate KC, Kraft G, Lautz M, Smith M, Neal DW, Cherrington AD (2011) A high-fat, high-fructose diet accelerates nutrient absorption and impairs net hepatic glucose uptake in response to a mixed meal in partially pancreatectomized dogs. J Nutr 141:1643–1651
Cormier H, Rudkowska I, Paradis AM, Thifault E, Garneau V, Lemieux S, Couture P, Vohl MC (2012) Association between polymorphisms in the fatty acid desaturase gene cluster and the plasma triacylglycerol response to an n-3 PUFA supplementation. Nutrients 4:1026–1041
De CR (2011) n-3 fatty acids in cardiovascular disease. N Engl J Med 364:2439–2450
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Fu D, Cong X, Ma Y, Cai H, Cai M, Li D, Lv M, Yuan X, Huang Y, Lv Z (2013) Genetic polymorphism of glucokinase on the risk of type 2 diabetes and impaired glucose regulation: evidence based on 298,468 subjects. PLoS One 8:e55727
Gao X, Starmer J, Martin ER (2008) A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol 32:361–369
Girard J, Ferre P, Foufelle F (1997a) Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu Rev Nutr 17:325–352
Girard J, Ferre P, Foufelle F (1997b) Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu Rev Nutr 17:325–352
Harris WS, Bulchandani D (2006) Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol 17:387–393
Hishida A, Morita E, Naito M, Okada R, Wakai K, Matsuo K, Nakamura K, Takashima N et al (2012) Associations of apolipoprotein A5 (APOA5), glucokinase (GCK) and glucokinase regulatory protein (GCKR) polymorphisms and lifestyle factors with the risk of dyslipidemia and dysglycemia in Japanese—a cross-sectional data from the J-MICC Study. Endocr J 59:589–599
Holmkvist J, Almgren P, Lyssenko V, Lindgren CM, Eriksson KF, Isomaa B, Tuomi T, Nilsson P, Groop L (2008) Common variants in maturity-onset diabetes of the young genes and future risk of type 2 diabetes. Diabetes 57:1738–1744
Hu C, Zhang R, Wang C, Yu W, Lu J, Ma X, Wang J, Jiang F, Tang S, Bao Y, Xiang K, Jia W (2010) Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLoS One 5:e11761
Iynedjian PB (2009) Molecular physiology of mammalian glucokinase. Cell Mol Life Sci 66:27–42
Iynedjian PB, Gjinovci A, Renold AE (1988) Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats. J Biol Chem 263:740–744
Jump DB, Clarke SD, Thelen A, Liimatta M (1994) Coordinate regulation of glycolytic and lipogenic gene expression by polyunsaturated fatty acids. J Lipid Res 35:1076–1084
Livak KJ (1999) Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 14:143–149
Madden J, Williams CM, Calder PC, Lietz G, Miles EA, Cordell H, Mathers JC, Minihane AM (2011) The impact of common gene variants on the response of biomarkers of cardiovascular disease (CVD) risk to increased fish oil fatty acids intakes. Annu Rev Nutr 31:203–234
Massa ML, Gagliardino JJ, Francini F (2011) Liver glucokinase: an overview on the regulatory mechanisms of its activity. IUBMB Life 63:1–6
McNamara JR, Schaefer EJ (1987) Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta 166:1–8
Minihane AM, Khan S, Leigh-Firbank EC, Talmud P, Wright JW, Murphy MC, Griffin BA, Williams CM (2000) ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arterioscler Thromb Vasc Biol 20:1990–1997
Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD (2012) Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr 3:286–294
Peter A, Stefan N, Cegan A, Walenta M, Wagner S, Konigsrainer A, Konigsrainer I, Machicao F, Schick F, Haring HU, Schleicher E (2011) Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. J Clin Endocrinol Metab 96:E1126–E1130
Rudkowska I, Paradis AM, Thifault E, Julien P, Barbier O, Couture P, Lemieux S, Vohl MC (2012) Differences in metabolomic and transcriptomic profiles between responders and non-responders to an n-3 polyunsaturated fatty acids (PUFAs) supplementation. Genes Nutr 8:411–423
Schwarz JM, Linfoot P, Dare D, Aghajanian K (2003) Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 77:43–50
Sotos-Prieto M, Guillen M, Vicente SJ, Portoles O, Guillem-Saiz P, Ignacio GJ, Qi L, Corella D (2013) Relevant associations of the glucokinase regulatory protein/glucokinase gene variation with TAG concentrations in a high-cardiovascular risk population: modulation by the Mediterranean diet. Br J Nutr 109:193–201
Thifault E, Cormier H, Bouchard-Mercier A, Rudkowska I, Paradis AM, Garneau V, Ouellette C, Lemieux S, Couture P, Vohl MC (2013) Effects of age, sex, body mass index and APOE genotype on cardiovascular biomarker response to an n-3 polyunsaturated fatty acid supplementation. J Nutrigenet Nutrigenomics 6:73–82
Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK (2006) Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res 47:2562–2574
Wang H, Liu L, Zhao J, Cui G, Chen C, Ding H, Wang DW (2013) Large scale meta-analyses of fasting plasma glucose raising variants in GCK, GCKR, MTNR1B and G6PC2 and their impacts on type 2 diabetes mellitus risk. PLoS One 8:e67665
Webster RJ, Warrington NM, Weedon MN, Hattersley AT, McCaskie PA, Beilby JP, Palmer LJ, Frayling TM (2009) The association of common genetic variants in the APOA5, LPL and GCK genes with longitudinal changes in metabolic and cardiovascular traits. Diabetologia 52:106–114
Acknowledgments
This research would not have been possible without the excellent collaboration of the participants. We would like to thank Hubert Cormier, Véronique Garneau, Alain Houde, Catherine Ouellette, Catherine Raymond, Élisabeth Thifault and the nurses, Danielle Aubin and Steeve Larouche, for their participation in the recruitment of the participants, the study coordination and the data collection. A.B.M. is supported by a studentship from the Fonds de recherche en santé du Quebec (FRQS) and by a Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Awards (201210GSD-304012-190387), I.R. is supported by a CIHR Bisby Postdoctoral Fellowship Award (200810BFE) and M.C.V. is Tier 1 Canada Research Chair in Genomics Applied to Nutrition and Health. This work was supported by a grant from CIHR—(MOP229488).
Conflict of interest
All the authors declare no conflict of interest.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bouchard-Mercier, A., Rudkowska, I., Lemieux, S. et al. An interaction effect between glucokinase gene variation and carbohydrate intakes modulates the plasma triglyceride response to a fish oil supplementation. Genes Nutr 9, 395 (2014). https://doi.org/10.1007/s12263-014-0395-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12263-014-0395-5