Abstract
GATA binding protein 3 was more sensitive than traditional markers such as gross cystic disease fluid protein 15 and mammaglobin for identifying primary and metastatic breast carcinomas, but its significance decreased in triple-negative breast cancer. Recent studies showed a high expression rate of proline glutamic acid and leucine-rich protein in breast cancer and their superiority over GATA3 in triple-negative breast cancer. Our study provided new insights into the diagnostic and prognostic roles of PELP1 and GATA3 in primary and metastatic breast cancer. An immunohistochemical assay was carried out using PELP1 and GATA3 in 60 cases of primary breast cancer and 15 metastatic. Invasive carcinoma of no special type was the predominant type (80%). The majority of cases were grade 3 (68.3%). GATA3 expression was 83.3% positive in primary breast carcinomas and 73.5% positive in metastatic breast carcinomas. In comparison, PELP1 had a 96.7% positive expression rate in primary breast carcinomas and an 86.7% positive expression rate in metastasis. There was a statistically significant agreement between GATA3 and PELP1 in the diagnosis of the cases. PELP1 is a significantly higher proportion of both primary and metastatic breast carcinomas than GATA3. In breast cancer, there was a strong association between favorable prognostic factors and GATA3 expression, with evidence of an inverse association with Ki-67 overexpression.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most frequent malignancy in women worldwide; it is the fifth leading cause of cancer mortality and the second leading cause of cancer death in women [1]. Despite the significant improvement in BC survival, distant metastases still represent major obstacles. Metastasis to vital organs has been identified as the principal cause of the majority of BC-related morbidity and mortality, as well as presenting diagnostic and therapeutic challenges [2].
The most widely used conventional membranous/cytoplasmic markers, mammaglobin (MGB) and gross cystic disease fluid protein 15 (GCDFP-15), are specific for the diagnosis of BC patients but have limited sensitivity and are difficult to interpret in small samples [3]. Novel diagnostic immunohistochemical markers with considerably increased sensitivity for primary and metastatic breast cancer (MBC), as well as the ability to predict therapy response and prospective target treatments, are urgently needed. GATA3 has been identified as a new marker for detecting primary and metastatic BC, with considerably more sensitivity than the conventional markers [4]. GATA3 is a zinc-binding transcription factor that controls the differentiation of various human tissue types, including the luminal epithelial cells of the breast [5, 6]. Also, understanding the role of GATA3 can be a promising potential target in BC management, as it was demonstrated as a requirement for estradiol stimulation for cell cycle progression of BC [7, 8]. However, GATA3 expression was still significantly decreased in triple-negative breast cancer (TNBC) [4] 9. Recent studies showed a high expression rate of proline glutamic acid and leucine-rich protein (PELP1) in BC and its superiority over GATA3 in identifying TNBC [10, 11].
PELP1 is a scaffolding protein that functions as a co-regulator of several transcription factors and multiple hormonal receptors and exhibits aberrant expression in many hormone-related cancers [12]. PELP1 overexpression had been shown to induce the malignant transformation of normal cells, accelerate cell cycle progression, modulate several signaling cascades, control the cell cytoskeleton, promote tumor cell proliferation, and promote the migration and metastases in BC [13, 14]. The current study provided novel insights into the diagnostic and prognostic role of PELP1 and GATA3 in different breast cancer subtypes by using immunohistochemistry as a practical and cost-effective method present in almost all laboratory centers, in contrast to molecular studies.
Patients and Methods
Patients and Tissue Specimens
From February 2019 to February 2021, 60 cases of selected woman patients with invasive breast carcinomas (IBC) were collected at the Pathology Department, Faculty of Medicine, Zagazig University. This study was performed after approval by the local ethical committee, Zagazig University, Institutional Review Board (IRB) for human studies (reference number is ZU-IRB #:5275–26-2-2019).
The specimens were obtained either by large needle core biopsy (n = 7, 2 of them from distant metastases), modified radical mastectomy (n = 50), and cell blocks from a pleural effusion (n = 3). The study also included 10 lymph nodes with metastasis (related to ten selected cases of BC). The clinicopathological data, which included patient age, tumor size, lymph node involvement, ER, PR, and Her2 status, and the ki67 (the cutoff point was set at 14% to distinguish high and low Ki-67) [15], were obtained from pathology reports that were included with the tissue specimens and also reports for MBC that had an available matched PBC specimen. Outside consult cases were excluded. Moderate-to-strong nuclear staining in 1% of tumor cells was considered positive for ER and PR expression. If complete, intense, circumferential membranous staining within >10% of tumor cells was found, HER2 was positive (according to ASCO/CAP HER2Testing Guideline Update, 2018) [16].
Histopathology and Immunohistochemistry Procedure
From the formalin-fixed, paraffin-embedded tissue blocks, 3–5 μm sections were cut for each case. Sections were stained with hematoxylin and eosin (H&E) and examined under light microscopy to confirm the diagnosis. The immunohistochemical assay was carried out using the polymer Envision Detection System, the Dako EnVision™ kit (Dako, Copenhagen, Denmark). The approved 3-in-1 specimen preparation approach was used for pretreatment. Formalin-fixed paraffin-embedded tissue sections were deparaffinized, rehydrated, and subjected to heat-induced epitope retrieval in preheated working solution of EnVision Target Retrieval Solution, Low pH, (code K8005, Agilent) in the PT Link Pretreatment module at 97 ℃ for 20 min then left to cool to 65 ℃. Sections were rinsed with EnVision TM Wash Buffer (DM 831, code K 8007, Agilent) in the PT Link Rinse Station (Agilent) for 5 min. Endogenous peroxidase activity was removed with EnVision Peroxidase-Blocking Reagent ready to use (SM801, Agilent) for 5 min at RT.
Then autostainer was used as follows: Primary antibodies a GATA3: a mouse monoclonal, clone L50-823, IgG1/kappa, RRID: AB_2895066 (CM 405 A, B, Biocare, USA), diluted 1:100; PELP1 rabbit polyclonal, clone A13414, IgG, RRID: AB_2760275 (Cat# A13414, ABclonal, USA), diluted 1:100. The reaction was visualized by incubating the sections with diaminobenzidine (DAB) for 15 min after that Mayer’s hematoxylin was used. GATA3 labeling was present in the benign luminal epithelial cells, and PELP1 labeling was in the endothelial cells and lymphocytes, serving as a positive internal control. Replacement of the primary antibody step with a blocking buffer was included in the staining procedure as a negative control.
Interpretation of Immunostaining
GATA3 and PELP1 immunoreactivity were evaluated independently in a blind manner by two pathologists using a binocular microscope (MC30, Micros, Austria) with a camera (MU1000A, Amscope, USA).
Evaluation of GATA3 Immunostaining
The intensity of the GATA3 nuclear labeling was scored as negative (0), weak (1 +), moderate (2 +), or strong (3 +) for association with clinicopathological parameters. The percentage of tumor cells was scored based on the extent of nuclear staining with a cutoff of 5%, defining GATA3 positive expression for the diagnosis of BC cases[17].
Evaluation of PELP1 Immunostaining
PELP1 staining was categorized into low (< 5%), focal (5–49%), and diffuse (≥ 50%). A cutoff of 5% was used to define PELP1 positivity for the diagnosis of BC cases [11].
Statistics
SPSS (Statistical Package for Social Science, Chicago, Illinois, USA) version 23 was used to collect, tabulate, and statistically analyze all data. We calculated mean and standard deviation for quantitative variables, while frequency and percentage were presented as qualitative variables. One-way ANOVA test was used to compare between more than two groups of normally distributed variables, while the Kruskal Wallis H test was used for non-normally distributed variables. The Chi-square test or Fisher’s exact test when was appropriate. Spearman’s correlation coefficient was applied to determine the correlation between the variables. P values less than 0.05 were considered statistically significant.
Results
The Clinicopathological Data of the Studied Cases
The clinicopathological data of the cases enrolled in this study were summarized. The age of patients in the studied cases at the time of initial diagnosis ranged from 25 to 72 years. The mean and median ages were 49.53 SD 12.64 years and 50 years, respectively. Invasive duct carcinoma of no special type was the most common histopathological type (80%). Most of the cases were grade 3 (68.3%). The most frequent molecular subtypes were luminal A and triple-negative (35% and 30%) (Table 1).
Clinicopathological Association of GATA3 Expression in Breast Cancer Cases
There was a statistically significant association between GATA3 expression and the age group above 35 years, lower grades, and absence of LVI (p-value 0.02, 0.02, 0.04). A statistically significant association between GATA3 expression and positive hormonal expression of ER and PR receptors, negative HER2, and low Ki-67 was noted (p-value < 0.001, < 0.001, 0.04, 0.001). Also, a significant GATA3 expression was found with luminal A and B molecular subtypes (p-value < 0.001) (Fig. 1; Table 2).
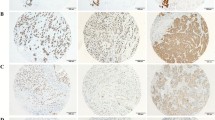
The immunohistochemical expression of GATA3 in study cases of different breast carcinoma types. (a) Invasive breast carcinoma (NST) (G 3) with LVI showing negative GATA3 expression, (b) mucinous breast carcinoma showing mild GATA3 expression, (c) lymph node metastatic from IBC NST showing moderate nuclear GATA3 expression, (d) papillary breast carcinoma showing high positive nuclear GATA3 expression, (scale bar = 40 µm)
Clinicopathological Association of PELP1 Expression in Breast Cancer Cases
In the present study, the immunohistochemical expression of PELP1 in tumor cells was positive in 58 out of 60 cases (96%). Moreover, the majority of the PELP1 positive cases showed diffuse strong staining with only 2 out of 58 cases showing focal staining. There was no statistical relation between PELP1 IHC expression and clinicopathological parameters (lymph node, EIS, skin invasion, lympho-vascular invasion, tumor-infiltrating lymphocytes, hormonal receptors, or molecular subtypes) except for BC histopathological types (lobular breast carcinoma) (Fig. 2) among the studied cases (Table 3).
The immunohistochemical expression of PELP1 in study cases of different breast carcinoma types. (a) Lymph node metastatic from IBC NST showing negative PELP1 expression. (b) Invasive carcinoma (NST) (G2) showing focal positive PELP1 expression. (c) Invasive carcinoma (NST) (G3) with LVI showing diffuse positive PELP1 expression, positive endothelial cells serve as a positive internal control. (d) Invasive lobular carcinoma showing strong diffuse nuclear positive PELP1 expression, (scale bar = 40 µm)
Relation Between PELP1 and GATA3 Expression
The immunohistochemical expression of PELP1 in tumor cells showed a (96.7%) positive expression rate in comparison to GATA3, which was found to be (83.3%) positive in BC cases. PLEP1 expression was (86.7%) positive in metastatic BC in comparison to GATA3, which was found to be (73%) positive in metastatic BC in the current study. There was a statistically significant agreement between GATA3 and PELP1 in the diagnosis of primary and metastatic breast cancer studied cases (Table 4).
Discussion
Although a metastatic tumor is late-stage cancer that is considered fatal, effective therapy can relieve tumor-related symptoms, delay cancer progression, prolong life, and enhance the quality of life [18, 19]. It makes a significant therapeutic and outcome difference when the primary organ of metastasis is well known [8]. Identification of breast differentiation in metastatic locations based on morphology is a diagnostic challenge [19, 20].
Recent studies showed a high expression rate of PELP1 in BC [10, 11] and its superiority over GATA3 in the diagnosis of TNBC [21]. These findings have prompted us to further evaluate PELP1 and GATA3 expression in different primary and metastatic BC molecular subtypes. In the current study, the most common histologic subtype is IBC NST (80%). This is consistent with all previously published papers on different types of BC [21, 23]. It is well known that BC is one of the hormone-dependent tumors, and its relationship with ER and PR has prognostic values [23]. In the present study, GATA3 labeling was seen overall in 83.3% (50/60) of cases, including 95.3% of luminal A, 91.7% of luminal B, 77.8% of Her-2 enriched carcinomas, and 66.7% of TNBCs. Most positive cases (55%) show strong staining. The intensity of staining ranged from moderate to strong in the luminal A and luminal B subgroups, and weak to moderate/strong in the HER-2 and triple-negative subgroups.
It is not unexpected that GATA3 staining is common in ER + breast carcinomas as GATA3 function is associated with the ER signaling pathway [24, 25]. GATA3 positivity was later reported to be present in 60–100 percent of ER+ woman primary BC [26,27,28,29,30,31,32,33,34,35,36,37]. GATA3’s association with luminal subtypes is consistent with its role as a master gene for luminal breast differentiation [37, 38]. Furthermore, a tumor-suppressive role of GATA3 in hormone-sensitive BC has been recognized [38]. In contrast to these results, only one research paper found 39% of ER + primary BC to be GATA3 positive [39]. Some papers reported decreased GATA3 expression in the luminal B subtype as determined by gene profiling [29].
We discovered a significant association between low GATA3 and HER-2 overexpression, which is consistent with previous research [40]. Furthermore, HER2-positive patients had a poor prognosis and lower disease-free survival [41]. Other studies, on the other hand, found no significant links between GATA3 and HER-2 [29, 37, 42]. The range in GATA3 positivity is impacted by the variability across the studies in specimen type, antibody choice, and diagnostic threshold for positivity. The nuclear labeling cutoffs used in the literature to define GATA3 positivity have ranged from any nuclear labeling [26] to 1% [28], to 5%[17, 32], and 30% [43]. In addition to labeling the majority of ER-positive BC, GATA3 also labels a subset of ER-negative primary BC with a wide range of reported positivity from 2.6 to 83% of IBC [6]. Of the two studies that have specifically evaluated the expression of GATA3 in TNBC, the reported range of GATA3 expression was 5% [44] and 16% [43], respectively. GATA3 expression was found in 69% (66/96) of ER-negative BC in a previous study [32]. This is consistent with our current study, which found GATA3 expression in 76.7% of TNBC.
Even though our presented work in this article and some previous works have demonstrated that GATA3 is expressed in TNBC, other murine models have suggested that GATA3 expression inhibits the triple-negative phenotype as presented in [45]. According to this study [46], loss of GATA3 expression is associated with negative ER status, and GATA3-negative tumors are enriched in the basal-like molecular subgroup.
Published data regarding GATA3 as a prognostic marker is conflicting. Loss of GATA3 expression has been associated with unfavorable clinical outcomes and worse survival [47]. However, no association with the outcome has been observed in other studies [48]. Looking at clinicopathological parameters, based on our findings, GATA3 (+) tumors were more likely to have a statistically significant increase in GATA3 expression in age group >35 years, low KI 67 (p < 0.001), and lower grades; GATA3 is directly associated with ER (p < 0.0001) and PR (p < 0.0001) expressions. Similarly, in studies [28, 37], those with GATA3 positive tumors were likelier to have lower-grade tumors. According to that paper [49], higher GATA3 expression is a good prognostic factor for overall survival in ER-positive BC patients.
Ki-67 is a proliferation marker that can only be detected in active cells, not in resting cells. In most cases, higher Ki-67 levels in breast cancer indicate a poor prognosis. This is consistent with our research. We discovered an inverse relationship between Ki-67 and GATA3 expression; similar findings have been reported by others [17, 50, 51]. Furthermore, no significant relationships were found between GATA3 expression and lymph node metastasis or tumor size [3, 39]. This finding is consistent with our findings, in contrast to other studies that discovered a significant relationship between GATA3 and tumor size [29, 37, 40]. GATA3 has been identified as a prognostic marker due to its ability to promote luminal progenitor cell differentiation [52]. These different results may be owing to different sensitivities of using different clones on breast resection tissues that have been documented in prior reports. Other contributing factors for expression disparities could also be related to tumor characteristics (grade and molecular subtypes) or technical causes (antigen retrieval procedures, dilutions, or incubation times) and different cohort study types. Our findings indicate that GATA3 is a positive prognostic marker in BC patients.
Recent research showed a high expression rate of PELP1 in BC and its superiority over GATA3 in identifying TNBC [10, 11]. In our study, PELP1 was exclusively nuclear in localization. This result is consistent with recent immunohistochemical studies using commercially available antibodies against PELP1 in a variety of tissues [53]. PELP1 staining was nuclear with no cytoplasmic staining in all BC molecular subtypes, as well as it is in some luminal ductal epithelial cells of associated normal tissues in the specimens.
The immunohistochemical expression of PELP1 in tumor cells was positive in 58 out of 60 cases (96%), and only 2 out of 58 show focal staining. Moreover, the majority of the PELP1 positive cases showed diffuse strong staining, making observation of the staining easy. These results were in line with Dang and colleagues, who found that 67 out of 70 primary BC (96%) had nuclear PELP1 staining [21]. In contrast to these results that were reported, 17.2% of the BC showed negative or low expression, 69.3% showed moderate expression, and 13.5% showed strong expression [53]. The reported discrepancy for PELP1 expression in these studies may reflect the different methodology used or the different cutoff values used to define PELP1 positivity.
A research study reported among 1063 BC cases with complete IHC data for molecular classification, a significant differential distribution of PELP1 expression was found, with diffuse staining mostly in luminal B (76.4%), followed by TNBC (72.7%), HER-2 (68.9%), and luminal A (63.7%) [11]. Conversely, in our present study, the highest expression is in luminal B and HER-2 (100%). As a prognostic marker in previous studies, PELP1 was associated with poor outcomes in luminal cancers [53] and in TNBC when combined with Ki67 [10]. We and others found no significant association between PELP1 protein expression and clinicopathological variables [10].
Immunohistochemistry is an essential component of diagnostic breast pathology. It assists in supporting the breast origin for primary or metastatic carcinomas and identifying non-mammary metastases to the breast. However, no single immunostaining marker is perfectly sensitive or specific [54]. Previous comparative studies demonstrated a higher expression rate of GATA3 (72–82.83%) than the traditional marker for identifying metastatic BC GCDFP-15 (44–62%) and MGB (36–64%) [3, 20, 36]. In the present study, GATA3 revealed an 83% positive expression rate in PBC and a 73% positive in metastatic. In comparison to PELP1, it revealed a (96.7%) positive expression rate in PBC and (86.7%) positive in metastatic. Only one study compared GATA3 and PELP1 IHC and discovered that PELP1 immunoreactivity was consistently maintained in paired primary and metastatic TNBC cases (100%) and was higher than GATA3 (40%) in the metastatic TNBC [21]. These findings suggest that PELP1 is a far more diagnostic marker than GATA3. PELP1 may be useful in diagnosing metastatic breast cancer in certain circumstances, such as a history of primary breast cancer in cases where other markers are negative.
Questions can be raised about the mechanism and molecular pathway for PELP1 protein overexpression in BC and maintenance in TNBC, as well as whether PELP1 can be used as a molecular target for TNBC therapy, which currently lacks targeted therapy. Thus, understanding the role of PELP1 in metastatic BC can be a promising potential target in BC management.
Finally, our study demonstrates significantly increased PELP1 protein expression in primary and metastatic BC as compared with GATA3, the most widely used in our practice for BC detection. There was a statistically significant agreement between PELP1 and GATA3 in the diagnosis of primary breast carcinoma (p-value 0.03) and metastatic breast cancer (p-value 0.04) in studied cases.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Ni YB, Tsang JYS, Shao MM et al (2018) GATA-3 is superior to GCDFP-15 and mammaglobin to identify primary and metastatic breast cancer. Breast Cancer Res Treat 169(1):25–32. https://doi.org/10.1007/s10549-017-4645-2
Yang Y, Lu S, Zeng W, Xie S, Xiao S (2017) GATA3 expression in clinically useful groups of breast carcinoma: a comparison with GCDFP15 and mammaglobin for identifying paired primary and metastatic tumors. Ann Diagn Pathol 26:1–5. https://doi.org/10.1016/j.anndiagpath.2016.09.011
Sangoi AR, Shrestha B, Yang G, Mego O, Beck AH (2016) The novel marker GATA3 is significantly more sensitive than traditional markers mammaglobin and GCDFP15 for identifying breast cancer in surgical and cytology specimens of metastatic and matched primary tumours. Appl Immunohistochem Mol Morphol 24(4):229–237. https://doi.org/10.1097/PAI.0000000000000186
Takaku M, Grimm SA, Wade PA (2015) GATA3 in breast cancer: tumor suppressor or oncogene? Gene Expr 16(4):163–168. https://doi.org/10.3727/105221615X14399878166113
Asch-Kendrick R, Cimino-Mathews A (2016) The role of GATA3 in breast carcinomas: a review. Hum Pathol 48:37–47. https://doi.org/10.1016/j.humpath.2015.09.035
Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M (2007) Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res 67(13):6477–6483. https://doi.org/10.1158/0008-5472.CAN-07-0746
Takaku M, Grimm SA, Roberts JD et al (2018) GATA3 zinc finger 2 mutations reprogram the breast cancer transcriptional network. Nat Commun 9(1):1059. https://doi.org/10.1038/s41467-018-03478-4
Laurent E, Begueret H, Bonhomme B et al (2019) SOX10, GATA3, GCDFP15, androgen receptor, and mammaglobin for the differential diagnosis between triple-negative breast cancer and TTF1-negative lung adenocarcinoma. Am J Surg Pathol 43(3):293–302. https://doi.org/10.1097/PAS.0000000000001216
Zhang Y, Dai J, McNamara KM et al (2015) Prognostic significance of proline, glutamic acid, leucine rich protein 1 (PELP1) in triple-negative breast cancer: a retrospective study on 129 cases. BMC Cancer 15:699. https://doi.org/10.1186/s12885-015-1694-y
Wang X, Tsang J, Lee MA et al (2019) The clinical value of PELP1 for breast cancer: a comparison with multiple cancers and analysis in breast cancer subtypes. Cancer Res Treat 51(2):706–717. https://doi.org/10.4143/crt.2018.316
Girard BJ, Daniel AR, Lange CA, Ostrander JH (2014) PELP1: a review of PELP1 interactions, signaling, and biology. Mol Cell Endocrinol 382(1):642–651. https://doi.org/10.1016/j.mce.2013.07.031
Ravindranathan P, Lange CA, Raj GV (2015) Minireview: deciphering the cellular functions of PELP1. Mol Endocrinol 29(9):1222–1229. https://doi.org/10.1210/me.2015-1049
Vallabhaneni S, Nair BC, Cortez V et al (2011) Significance of ER-Src axis in hormonal therapy resistance. Breast Cancer Res Treat 130(2):377–385. https://doi.org/10.1007/s10549-010-1312-2
Gnant M, Harbeck N, Thomssen CS, Gallen, (2011) Summary of the consensus discussion. Breast Care (Basel) 6(2):136–141. https://doi.org/10.1159/000328054
Wolff AC, Hammond MEH, Allison KH et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142:1364–1382
Yildirim E, Bektas S, Gundogar O et al (2020) The relationship of GATA3 and Ki-67 with histopathological prognostic parameters, locoregional recurrence and disease-free survival in invasive ductal carcinoma of the breast. Anticancer Res 40(10):5649–5657. https://doi.org/10.21873/anticanres.14578
Riggio AI, Varley KE, Welm AL (2021) The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer 124(1):13–26. https://doi.org/10.1038/s41416-020-01161-4
Hafez NH, Shaaban HM (2018) Can GATA3 Immunocytochemistry be utilized as a reliable diagnostic marker for metastatic breast carcinoma in cytological materials? A comparative study with mammaglobin and GCDFP-15 expression. Turk Patoloji Derg 34(2):143–149. https://doi.org/10.5146/tjpath.2017.01419
Shaoxian T, Baohua Y, Xiaoli X et al (2017) Characterisation of GATA3 expression in invasive breast cancer: differences in histological subtypes and immunohistochemically defined molecular subtypes. J Clin Pathol 70(11):926–934. https://doi.org/10.1136/jclinpath-2016-204137
Dang DN, Raj G, Sarode V, Molberg KH, Vadlamudi RK, Peng Y (2015) Significantly increased PELP1 protein expression in primary and metastatic triple-negative breast carcinoma: comparison with GATA3 expression and PELP1’s potential role in triple-negative breast carcinoma. Hum Pathol 46(12):1829–1835. https://doi.org/10.1016/j.humpath.2015.07.023
Guan X, Xu G, Shi A et al (2020) Comparison of clinicopathological characteristics and prognosis among patients with pure invasive ductal carcinoma, invasive ductal carcinoma coexisted with invasive micropapillary carcinoma, and invasive ductal carcinoma coexisted with ductal carcinoma in situ: a retrospective cohort study. Medicine (Baltimore) 99(50):e23487. https://doi.org/10.1097/MD.0000000000023487
Badowska-Kozakiewicz AM, Liszcz A, Sobol M, Patera J (2017) Retrospective evaluation of histopathological examinations in invasive ductal breast cancer of no special type: an analysis of 691 patients. Arch Med Sci 13(6):1408–1415. https://doi.org/10.5114/aoms.2015.53964
Wilson BJ, Giguère V (2008) Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Mol Cancer 7:49. https://doi.org/10.1186/1476-4598-7-49
Thakkar A, Raj H, Ravishankar MB, Balakrishnan A, Padigaru M (2015) High expression of three-gene signature improves prediction of relapse-free survival in estrogen receptor-positive and node-positive breast tumors. Biomark Insights 10:103–112. https://doi.org/10.4137/BMI.S30559
Liu H, Shi J, Wilkerson ML, Lin F (2012) Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol 138(1):57–64. https://doi.org/10.1309/AJCP5UAFMSA9ZQBZ
Miettinen M, McCue PA, Sarlomo-Rikala M et al (2014) GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol 38(1):13–22. https://doi.org/10.1097/PAS.0b013e3182a0218f
Gulbahce HE, Sweeney C, Surowiecka M, Knapp D, Varghese L, Blair CK (2013) Significance of GATA-3 expression in outcomes of patients with breast cancer who received systemic chemotherapy and/or hormonal therapy and clinicopathologic features of GATA-3-positive tumors. Hum Pathol 44(11):2427–2431. https://doi.org/10.1016/j.humpath.2013.05.022
Tominaga N, Naoi Y, Shimazu K et al (2012) Clinicopathological analysis of GATA3-positive breast cancers with special reference to response to neoadjuvant chemotherapy. Ann Oncol 23(12):3051–3057. https://doi.org/10.1093/annonc/mds120
Gonzalez RS, Wang J, Kraus T, Sullivan H, Adams AL, Cohen C (2013) GATA-3 expression in male and female breast cancers: comparison of clinicopathologic parameters and prognostic relevance. Hum Pathol 44(6):1065–1070. https://doi.org/10.1016/j.humpath.2012.09.010
Cimino-Mathews A, Subhawong AP, Illei PB et al (2013) GATA3 expression in breast carcinoma: utility in triple-negative, sarcomatoid, and metastatic carcinomas. Hum Pathol 4 4(7):1341–1349. https://doi.org/10.1016/j.humpath.2012.11.003
Liu H, Shi J, Prichard JW, Gong Y, Lin F (2014) Immunohistochemical evaluation of GATA-3 expression in ER-negative breast carcinomas. Am J Clin Pathol 141(5):648–655. https://doi.org/10.1309/AJCP0Q9UQTEESLHN
Clark BZ, Beriwal S, Dabbs DJ, Bhargava R (2014) Semiquantitative GATA-3 immunoreactivity in breast, bladder, gynecologic tract, and other cytokeratin 7-positive carcinomas. Am J Clin Pathol 142(1):64–71. https://doi.org/10.1309/AJCP8H2VBDSCIOBF
Ordóñez NG, Sahin AA (2014) Diagnostic utility of immunohistochemistry in distinguishing between epithelioid pleural mesotheliomas and breast carcinomas: a comparative study. Hum Pathol 45(7):1529–1540. https://doi.org/10.1016/j.humpath.2014.03.006
Krings G, Nystrom M, Mehdi I, Vohra P, Chen YY (2014) Diagnostic utility and sensitivities of GATA3 antibodies in triple-negative breast cancer. Hum Pathol 45(11):2225–2232. https://doi.org/10.1016/j.humpath.2014.06.022
Wendroth SM, Mentrikoski MJ, Wick MR (2015) GATA3 expression in morphologic subtypes of breast carcinoma: a comparison with gross cystic disease fluid protein 15 and mammaglobin. Ann Diagn Pathol 19(1):6–9. https://doi.org/10.1016/j.anndiagpath.2014.12.001
Guo Y, Yu P, Liu Z et al (2017) Prognostic and clinicopathological value of GATA binding protein 3 in breast cancer: a systematic review and meta-analysis. PLoS ONE 12(4):e0174843. https://doi.org/10.1371/journal.pone.0174843
Lin HY, Zeng D, Liang YK, Wei XL, Chen CF (2017) GATA3 and TRPS1 are distinct biomarkers and prognostic factors in breast cancer: database mining for GATA family members in malignancies. Oncotarget. 8(21):34750–34761. https://doi.org/10.18632/oncotarget.16160
Voduc D, Cheang M, Nielsen T (2008) GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol Biomarkers Prev 17(2):365–373. https://doi.org/10.1158/1055-9965.EPI-06-1090
Mehra R, Varambally S, Ding L et al (2005) Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res 65(24):11259–11264. https://doi.org/10.1158/0008-5472.CAN-05-2495
Siadati S, Sharbatdaran M, Nikbakhsh N, Ghaemian N (2015) Correlation of ER, PR and HER-2/Neu with other prognostic factors in infiltrating ductal carcinoma of breast. Iran J Pathol 10(3):221–226
Hisamatsu Y, Tokunaga E, Yamashita N et al (2015) Impact of GATA-3 and FOXA1 expression in patients with hormone receptor-positive/HER2-negative breast cancer. Breast Cancer 22(5):520–528. https://doi.org/10.1007/s12282-013-0515-x
Albergaria A, Paredes J, Sousa B et al (2009) Expression of FOXA1 and GATA-3 in breast cancer: the prognostic significance in hormone receptor-negative tumours. Breast Cancer Res 11(3):R40. https://doi.org/10.1186/bcr2327
Yang M, Nonaka D (2010) A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol 23(5):654–661. https://doi.org/10.1038/modpathol.2010.38
Chu IM, Michalowski AM, Hoenerhoff M et al (2012) GATA3 inhibits lysyl oxidase-mediated metastases of human basal triple-negative breast cancer cells. Oncogene 31(16):2017–2027. https://doi.org/10.1038/onc.2011.382
Lu S, Yakirevich E, Wang LJ, Resnick MB, Wang Y (2019) Cytokeratin 7-negative and GATA binding protein 3-negative breast cancers: clinicopathological features and prognostic significance. BMC Cancer 19(1):1085. https://doi.org/10.1186/s12885-019-6295-8
McCleskey BC, Penedo TL, Zhang K, Hameed O, Siegal GP, Wei S (2015) GATA3 expression in advanced breast cancer: prognostic value and organ-specific relapse. Am J Clin Pathol 144(5):756–763. https://doi.org/10.1309/AJCP5MMR1FJVVTPK
Ni YB, Tsang JY, Chan SK, Tse GM (2015) GATA-binding protein 3, gross cystic disease fluid protein-15 and mammaglobin have distinct prognostic implications in different invasive breast carcinoma subgroups. Histopathology 67(1):96–105. https://doi.org/10.1111/his.12625
Fararjeh AS, Tu SH, Chen LC et al (2018) The impact of the effectiveness of GATA3 as a prognostic factor in breast cancer. Hum Pathol 80:219–230. https://doi.org/10.1016/j.humpath.2018.06.004
Kim S, Moon BI, Lim W, Park S, Cho MS, Sung SH (2016) Expression patterns of GATA3 and the androgen receptor are strongly correlated in patients with triple-negative breast cancer. Hum Pathol 55:190–195. https://doi.org/10.1016/j.humpath.2016.04.013
Dydensborg AB, Rose AA, Wilson BJ et al (2009) GATA3 inhibits breast cancer growth and pulmonary breast cancer metastasis. Oncogene 28(29):2634–2642. https://doi.org/10.1038/onc.2009.126
Cakir A, Isik Gonul I, Ekinci O, Cetin B, Benekli M, Uluoglu O (2017) GATA3 expression and its relationship with clinicopathological parameters in invasive breast carcinomas. Pathol Res Pract 213(3):227–234. https://doi.org/10.1016/j.prp.2016.12.010
Habashy HO, Powe DG, Rakha EA et al (2010) The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat 120(3):603–612. https://doi.org/10.1007/s10549-009-0419-9
Cimino-Mathews A (2021) Novel uses of immunohistochemistry in breast pathology: interpretation and pitfalls. Modern pathol: an official J United States and Canadian Acad Pathol 34(Suppl 1):62–77. https://doi.org/10.1038/s41379-020-00697-3
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors confirm responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
Corresponding author
Ethics declarations
Ethics Approval
The Institutional Review Board (IRB) of the Faculty of Medicine at Zagazig University confirmed the study protocol. The study was carried out at the Pathology Department, Faculty of Medicine, Zagazig University.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moustafa, M., Ismael, M., Mohamed, S. et al. Value of Proline, Glutamic Acid, and Leucine-Rich Protein 1 and GATA Binding Protein 3 Expression in Breast Cancer: An Immunohistochemical study. Indian J Surg 85, 608–617 (2023). https://doi.org/10.1007/s12262-022-03535-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-022-03535-9