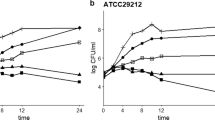
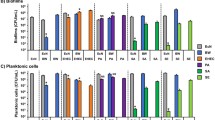
Abstract
Enterohemorrhagic Escherichia coli O157:H7 (EHEC) is responsible for outbreaks of hemorrhagic colitis worldwide, but no effective therapy exists for EHEC infections. EHEC readily forms antimicrobial-tolerant biofilms on various biotic and abiotic surfaces. Here, we investigated the antimicrobial and antibiofilm abilities of 16 halogenated (fluoro-, chloro-, bromo-, or iodo-) indoles and indole against a pathogenic EHEC strain. Antibiofilm activities followed the order chloroindoles > bromoindoles > indole > fluoroindoles. For example, the minimum inhibitory concentrations (MICs) of 4-bromoindole and 5-bromoindole were 100 and 200 μg/mL, respectively, and at 20 μg/mL, they both inhibited EHEC biofilm formation by more than 61% without affecting planktonic cell growth. However, at concentrations greater than their MICs, both showed bactericidal activity. Antibiofilm effects were confirmed by scanning electron microscopy. Both 4-bromoindole and 5-bromoindole reduced swimming and swarming motility and curli formation, which are important factors for EHEC biofilm formation. Furthermore, quantitative structure–activity relationship analysis demonstrated that halogenation of indole with chlorine, bromine, or iodine at positions C-4 or C-5 promotes antimicrobial activity but that substitution at C-7 is detrimental. The study shows that halogenated indoles, particularly bromoindoles, have potential use as antimicrobial and antibiofilm therapies against EHEC.






Similar content being viewed by others
References
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS Suppl 136:1–51. https://doi.org/10.1111/apm.12099
Raj DS, Dhamodharan D, Thanigaivel S et al (2022) Nanoemulsion as an effective inhibitor of biofilm-forming bacterial associated drug resistance: an insight into COVID based nosocomial infections. Biotechnol Bioprocess Eng 27:543–555. https://doi.org/10.1007/s12257-022-0055-3
Nataro JP, Kaper JB (1998) Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201. https://doi.org/10.1128/CMR.11.1.142
Tarr PI, Gordon CA, Chandler WL (2005) Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086. https://doi.org/10.1016/S0140-6736(05)71144-2
Patel J, Sharma M, Ravishakar S (2011) Effect of curli expression and hydrophobicity of Escherichia coli O157:H7 on attachment to fresh produce surfaces. J Appl Microbiol 110:737–745. https://doi.org/10.1111/j.1365-2672.2010.04933.x
Ryu JH, Beuchat LR (2005) Biofilm formation by Escherichia coli O157:H7 on stainless steel: effect of exopolysaccharide and curli production on its resistance to chlorine. Appl Environ Microbiol 71:247–254. https://doi.org/10.1128/AEM.71.1.247-254.2005
Wood TK (2009) Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ Microbiol 11:1–15. https://doi.org/10.1111/j.1462-2920.2008.01768.x
Worthington RJ, Richards JJ, Melander C (2012) Small molecule control of bacterial biofilms. Org Biomol Chem 10:7457–7474. https://doi.org/10.1039/c2ob25835h
Lee J-H, Lee J (2010) Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. https://doi.org/10.1111/j.1574-6976.2009.00204.x
Lee J-H, Wood TK, Lee J (2015) Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol 23:707–718. https://doi.org/10.1016/j.tim.2015.08.001
Kumar P, Lee J-H, Lee J (2021) Diverse roles of microbial indole compounds in eukaryotic systems. Biol Rev Camb Philos Soc 96:2522–2545. https://doi.org/10.1111/brv.12765
Bansal T, Englert D, Lee J et al (2007) Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun 75:4597–4607. https://doi.org/10.1128/IAI.00630-07
Lee J, Bansal T, Jayaraman A et al (2007) Enterohemorrhagic Escherichia coli biofilms are inhibited by 7-hydroxyindole and stimulated by isatin. Appl Environ Microbiol 73:4100–4109. https://doi.org/10.1128/AEM.00360-07
Lee J-H, Cho MH, Lee J (2011) 3-indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ Microbiol 13:62–73. https://doi.org/10.1111/j.1462-2920.2010.02308.x
Lee J-H, Kim Y-G, Kim CJ et al (2012) Indole-3-acetaldehyde from Rhodococcus sp. BFI 332 inhibits Escherichia coli O157:H7 biofilm formation. Appl Microbiol Biotechnol 96:1071–1078. https://doi.org/10.1007/s00253-012-3881-y
Lee J-H, Kim Y-G, Cho MH et al (2012) 7-fluoroindole as an antivirulence compound against Pseudomonas aeruginosa. FEMS Microbiol Lett 329:36–44. https://doi.org/10.1111/j.1574-6968.2012.02500.x
Lee J-H, Kim Y-G, Gwon G et al (2016) Halogenated indoles eradicate bacterial persister cells and biofilms. AMB Express 6:123. https://doi.org/10.1186/s13568-016-0297-6
Manoharan RK, Lee J-H, Lee J (2018) Efficacy of 7-benzyloxyindole and other halogenated indoles to inhibit Candida albicans biofilm and hyphal formation. Microb Biotechnol 11:1060–1069. https://doi.org/10.1111/1751-7915.13268
Raorane CJ, Lee J-H, Lee J (2020) Rapid killing and biofilm inhibition of multidrug-resistant Acinetobacter baumannii strains and other microbes by iodoindoles. Biomolecules 10:1186. https://doi.org/10.3390/biom10081186
Sethupathy S, Sathiyamoorthi E, Kim Y-G et al (2020) Antibiofilm and antivirulence properties of indoles against Serratia marcescens. Front Microbiol 11:584812. https://doi.org/10.3389/fmicb.2020.584812
Sathiyamoorthi E, Faleye OS, Lee J-H et al (2021) Antibacterial and antibiofilm activities of chloroindoles against Vibrio parahaemolyticus. Front Microbiol 12:714371. https://doi.org/10.3389/fmicb.2021.714371
Zhang S, Yang Q, Defoirdt T (2022) Halogenated indoles decrease the virulence of Vibrio campbellii in a gnotobiotic brine shrimp model. Microbiol Spectr 10:e0268922. https://doi.org/10.1128/spectrum.02689-22
Ahmed B, Jailani A, Lee J-H et al (2022) Effect of halogenated indoles on biofilm formation, virulence, and root surface colonization by Agrobacterium tumefaciens. Chemosphere 293:133603. https://doi.org/10.1016/j.chemosphere.2022.133603
Boya BR, Lee J-H, Lee J (2022) Antibiofilm and antimicrobial activities of chloroindoles against uropathogenic Escherichia coli. Front Microbiol 13:872943. https://doi.org/10.3389/fmicb.2022.872943
Faleye OS, Boya BR, Lee JH et al (2023) Halogenated antimicrobial agents to combat drug-resistant pathogens. Pharmacol Rev 76:90–141. https://doi.org/10.1124/pharmrev.123.000863
Kim Y-G, Lee J-H, Gwon G et al (2016) Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci Rep 6:36377. https://doi.org/10.1038/srep36377
Clatworthy AE, Pierson E, Hung DT (2007) Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 3:541–548. https://doi.org/10.1038/nchembio.2007.24
Pratt LA, Kolter R (1998) Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol 30:285–293. https://doi.org/10.1046/j.1365-2958.1998.01061.x
Ren D, Sims JJ, Wood TK (2001) Inhibition of biofilm formation and swarming of Escherichia coli by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Environ Microbiol 3:731–736. https://doi.org/10.1046/j.1462-2920.2001.00249.x
Chapman MR, Robinson LS, Pinkner JS et al (2002) Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851–855. https://doi.org/10.1126/science.1067484
Verma J, Khedkar VM, Coutinho EC (2010) 3D-QSAR in drug design–a review. Curr Top Med Chem 10:95–115. https://doi.org/10.2174/156802610790232260
Zhang H, Shen C, Zhang HR et al (2021) Discovery of novel DGAT1 inhibitors by combination of machine learning methods, pharmacophore model and 3D-QSAR model. Mol Divers 25:1481–1495. https://doi.org/10.1007/s11030-021-10247-x
Ren JX, Zhang RT, Zhang H (2020) Identifying novel ATX inhibitors via combinatory virtual screening using crystallography-derived pharmacophore modelling, docking study, and QSAR analysis. Molecules 25:1107. https://doi.org/10.3390/molecules25051107
Mali SN, Pandey A, Bhandare RR et al (2022) Identification of hydantoin based Decaprenylphosphoryl-β-D-Ribose Oxidase (DprE1) inhibitors as antimycobacterial agents using computational tools. Sci Rep 12:16368. https://doi.org/10.1038/s41598-022-20325-1
Boya BR, Lee J-H, Lee J (2024) Antimicrobial and antibiofilm activities of chromone derivatives against uropathogenic Escherichia coli. Microbiol Res 278:127537. https://doi.org/10.1016/j.micres.2023.127537
Hadni H, Elhallaoui M (2020) 2D and 3D-QSAR, molecular docking and ADMET properties in silico studies of azaaurones as antimalarial agents. New J Chem 44:6553–6565. https://doi.org/10.1039/C9NJ05767F
Cavallo G, Metrangolo P, Milani R et al (2016) The halogen bond. Chem Rev 116:2478–2601. https://doi.org/10.1021/acs.chemrev.5b00484
Acknowledgements
This study was supported by a Yeungnam University Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Neither ethical approval nor informed consent was required for this study.
Informed consent
Jintae Lee is an Editorial Board Member for Biotechnology and Bioprocess Engineering and was not involved in the editorial review or the decision to publish this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeon, H., Boya, B.R., Kim, G. et al. Inhibitory effects of bromoindoles on Escherichia coli O157:H7 biofilms. Biotechnol Bioproc E (2024). https://doi.org/10.1007/s12257-024-00097-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12257-024-00097-3