Abstract
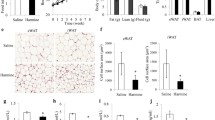
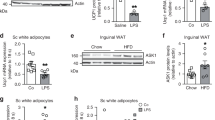
Increasing the number of brite cells (browning) in white adipocytes has attracted considerable attention to combat obesity because brite cells also help elevate energy expenditure. Sodium-potassium adenosine triphosphatase α2 subunit (ATP1A2) has been studied extensively in migraine and cancers. On the other hand, the role of ATP1A2 in adipocytes biology with a focus on fat browning needs to be elucidated. In this study, suppression of ATP1A2 induced browning in white adipocytes. The siRNA-mediated knockdown was used to identify the functional roles of the ATP1A2 gene in white adipocytes browning and the lipid metabolism. A deficiency of ATP1A2 promoted the expression of brown adipocyte-specific proteins and genes, suppressed adipogenesis and lipogenesis, and enhanced lipolysis and fat oxidation, as well as mitochondrial biogenesis. Moreover, silencing of ATP1A2 enhanced the expression of marker proteins for UCPl-dependent (β3-AR, PKA, p38, ATF2, and ERK) and UCP1-independent (α1-AR, SERCA, and RyR) thermogenesis. A mechanistic study showed that a deficiency of ATP1A2 induces browning in white adipocytes by activating the β3-AR/ERK signaling pathways as well as α1-AR/SERCA-based thermogenesis through an ATP-consuming process. In conclusion, ATP1A2 is a previously unrecognized player in thermogenesis in white adipocytes, and downregulating ATP1A2 and activating both UCP1-dependent and UCP1-independent thermogenesis in adipocytes could be a novel pharmacotherapeutic approach to treat obesity.
Similar content being viewed by others
References
Dragano, N. R. V., J. Fernø, C. Diéguez, M. López, and E. Milbank (2020) Recent updates on obesity treatments: available drugs and future directions. Neuroscience 437: 215–239.
Harms, M. and P. Seale (2013) Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19: 1252–1263.
Leitner, B. P., S. Huang, R. J. Brychta, C. J. Duckworth, A. S. Baskin, S. McGehee, I. Tal, W. Dieckmann, G. Gupta, G. M. Kolodny, K. Pacak, P. Herscovitch, A. M. Cypess, and K. Y Chen (2017) Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. U. S. A. 114: 8649–8654.
Kajimura, S., B. M. Spiegelman, and P. Seale (2015) Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22: 546–559.
Villarroya, F., R. Cereijo, J. Villarroya, and M. Giralt (2017) Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 13: 26–35.
Klepac, K., A. Georgiadi, M. Tschöp, and S. Herzig (2019) The role of brown and beige adipose tissue in glycaemic control. Mol. Aspects Med. 68: 90–100.
Ukropec J., R. P. Anunciado, Y. Ravussin, M. W. Hulver, and L. P. Kozak (2006) UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J. Biol. Chem. 281: 31894–31908.
Brownstein A. J., M. Veliova, R. Acin-Perez, M. Liesa, and O. S. Shirihai (2022) ATP-consuming futile cycles as energy dissipating mechanisms to counteract obesity. Rev. Endocr. Metab. Disord. 23: 121–131.
Ikeda K., Q. Kang, T. Yoneshiro, J. P. Camporez, H. Maki, M. Homma, K. Shinoda, Y. Chen, X. Lu, P. Maretich, K. Tajima, K. M. Ajuwon, T. Soga, and S. Kajimura (2017) UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23: 1454–1465.
Choi M., S. Mukherjee, and J. W. Yun (2022) Curcumin stimulates UCP1-independent thermogenesis in 3T3-L1 white adipocytes but suppresses in C2C12 muscle cells. Biotechnol. Bioprocess Eng. 27: 961–974.
Mukherjee S., M. Choi, and J. W. Yun (2022) Trans-anethole induces thermogenesis via activating SERCA/SLN axis in C2C12 muscle cells. Biotechnol. Bioprocess Eng. 27: 938–948.
Pradhan R. N., J. J. Bues, V. Gardeux, P. C. Schwalie, D. Alpern, W. Chen, J. Russeil, S. K. Raghav, and B. Deplancke (2017) Dissecting the brown adipogenic regulatory network using integrative genomics. Sci. Rep. 7: 42130.
Shapira, S. N. and P. Seale (2019) Transcriptional control of brown and beige fat development and function. Obesity (Silver Spring) 27: 13–21.
Subramani, M. and J. W. Yun (2021) Loss of lymphocyte cytosolic protein 1 (LCP1) induces browning in 3T3-L1 adipocytes via β3-AR and the ERK-independent signaling pathway. Int. J. Biochem. Cell Biol. 138: 106053.
Manigandan, S. and J. W. Yun (2022) Loss of cytoplasmic FMR1-interacting protein 2 (CYFIP2) induces browning in 3T3-L1 adipocytes via repression of GABA-BR and activation of mTORC1. J. Cell Biochem. 123: 863–877.
Kapri-Pardes, E., A. Katz, H. Haviv, Y. Mahmmoud, M. Ilan, I. Khalfin-Penigel, S. Carmeli, O. Yarden, and S. J. D. Karlish (2011) Stabilization of the α2 isoform of Na, K-ATPase by mutations in a phospholipid binding pocket. J. Biol. Chem. 286: 42888–42899.
Sodhi, K., J. Denvir, J. Liu, J. R. Sanabria, Y. Chen, R. Silverstein, Z. Xie, N. G. Abraham, and J. I. Shapiro (2020) Oxidant-induced alterations in the adipocyte transcriptome: role of the Na,K-ATPase oxidant amplification loop. Int. J. Mol. Sci. 21: 5923.
Blanco, G. and R. W. Mercer (1998) Isozymes of the Na/K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275: F633–F650.
Mobasheri, A., J. Avila, I. Cózar-Castellano, M. D. Brownleader, M. Trevan, M. J. Francis, J. F. Lamb, and P. Martín-Vasallo (2000) Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci. Rep. 20: 51–91.
Matchkov, V. V. and I. I. Krivoi (2016) Specialized functional diversity and interactions of the Na,K-ATPase. Front. Physiol. 7: 179.
Golovina, V. A., H. Song, P. F. James, J. B. Lingrel, and M. P. Blaustein (2003) Na+ pump alpha 2-subunit expression modulates Ca2+ signaling. Am. J. Physiol. Cell Physiol. 284: C475–C486.
Matchkov, V. V., N. Moeller-Nielsen, V. S. Dam, Z. Nourian, D. M. Briggs Boedtkjer, and C. Aalkjaer (2012) The α2 isoform of the Na,K-pump is important for intercellular communication, agonist-induced contraction, and EDHF-like response in rat mesenteric arteries. Am. J. Physiol. Heart Cire. Physiol. 303: H36–H46.
Mosmann, T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65: 55–63.
Wang, L., F. Xu, X.-J. Zhang, R.-M. Jin, and X. Li (2015) Effect of high-fat diet on cholesterol metabolism in rats and its association with Na+/K+-ATPase/Src/pERK signaling pathway. J. Huazhong Univ. Sci. Technolog. Med. Sci. 35: 490–494.
Cornelius, F., M. Habeck, R. Kanai, C. Toyoshima, and S. J. D. Karlish (2015) General and specific lipid–protein interactions in Na,K-ATPase. Biochim. Biophys. Acta 1848: 1729–1743.
Xie, Z.-J., J. Novograd, Y. Itzkowitz, A. Sher, Y. D. Buchen, K. Sodhi, N. G. Abraham, and J. I. Shapiro (2020) The pivotal role of adipocyte-Na K peptide in reversing systemic inflammation in obesity and COVID-19 in the development of heart failure. Antioxidants (Basel) 9: 1129.
Yang, W., T. Kelly, and J. He (2007) Genetic epidemiology of obesity. Epidemiol. Rev. 29: 49–61.
Bonilauri, B., A. C. Camillo-Andrade, M. D. M. Santos, J. S. D. G. Fischer, P. C. Carvalho, and B. Dallagiovanna (2021) Proteogenomic analysis reveals proteins involved in the first step of adipogenesis in human adipose-derived stem cells. Stem Cells Int. 2021: 3168428.
Russo, J. J. and K. J. Sweadner (1993) Na(+)-K(+)-ATPase subunit isoform pattern modification by mitogenic insulin concentration in 3T3-L1 preadipocytes. Am. J. Physiol. 264: C311–C316.
Obradovic, M., S. Zafirovic, A. Jovanovic, E. S. Milovanovic, S. A. Mousa, M. Labudovic-Borovic, and E. R. Isenovic (2015) Effects of 17β-estradiol on cardiac Na(+)/K(+)-ATPase in high fat diet fed rats. Mol. Cell. Endocrinol. 416: 46–56.
Gusarova, G. A., H. E. Trejo, L. A. Dada, A. Briva, L. C. Welch, R. B. Hamanaka, G. M. Mutlu, N. S. Chandel, M. Prakriya, and J. I. Sznajder (2011) Hypoxia leads to Na,K-ATPase downregulation via Ca(2+) release-activated Ca(2+) channels and AMPK activation. Mol. Cell. Biol. 31: 3546–3556.
Bray, G. A. and Y. Yukimura (1978) Activity of (Na+ + K+)-ATPase in the liver of animals with experimental obesity. Life Sci. 22: 1637–1642.
Mimura, M., H. Makino, A. Kanatsuka, T. Asai, and S. Yoshida (1994) Reduction of erythrocyte (Na(+)-K+)ATPase activity in type 2 (non-insulin-dependent) diabetic patients with micro-albuminuria. Horm. Metab. Res. 26: 33–38.
Sennoune, S., A. Gerbi, M. J. Duran, J. P. Grillasca, E. Compe, S. Pierre, R. Planells, M. Bourdeaux, P. Vague, G. Pieroni, and J. M. Maixent (2000) Effect of streptozotocin-induced diabetes on rat liver Na+/K+-ATPase. Eur. J. Biochem. 267: 2071–2078.
Scherzer, P., I. Nachliel, H. Bar-On, M. M. Popovtzer, and E. Ziv (2000) Renal Na-K-ATPase hyperactivity in diabetic Psammomys obesus is related to glomerular hyperfiltration but is insulin-independent. J. Endocrinol. 167: 347–354.
Kawakami, K., T. Onaka, M. Iwase, I. Homma, and K. Ikeda (2005) Hyperphagia and obesity in Na,K-ATPase alpha2 subunit-defective mice. Obes. Res. 13: 1661–1671.
Rothwell, N. J., M. E. Saville, M. J. Stock, and M. G. Wyllie (1982) Catecholamine and thyroid hormone influence on brown fat Na+, K+-ATPase activity and thermogenesis in the rat. Horm. Metab. Res. 14: 261–265.
Clausen, T. (2003) Na+-K+ pump regulation and skeletal muscle contractility. Physiol. Rev. 83: 1269–1324.
Beltowski, J., A. Jamroz-Wiœniewska, E. Borkowska, J. Nazar, and A. Marciniak (2005) Antioxidant treatment normalizes renal Na+,K+-ATPase activity in leptin-treated rats. Pharmaeol. Rep. 57: 219–228.
Nisoli, E., E. Clementi, C. Paolucci, V. Cozzi, C. Tonello, C. Sciorati, R. Bracale, A. Valerio, M. Francolini, S. Moncada, and M. O. Carruba (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899.
Gusarova, G. A., L. A. Dada, A. M. Kelly, C. Brodie, L. A. Witters, N. S. Chandel, and J. I. Sznajder (2009) Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol. Cell. Biol. 29: 3455–3464.
Lee, S. C., R. Nuccitelli, and P. A. Pappone (1993) Adrenergically activated Ca2+ increases in brown fat cells: effects of Ca2+, K+, and K channel block. Am. J. Physiol. 264: C217–C228.
Gao, J., R. Wymore, R. T. Wymore, Y. Wang, D. McKinnon, J. E. Dixon, R. T. Mathias, I. S. Cohen, and G. J. Baldo (1999) Isoform-specific regulation of the sodium pump by alpha- and beta-adrenergic agonists in the guinea-pig ventricle. J. Physiol. 516: 377–383.
Matos, M., E. Augusto, P. Agostinho, R. A. Cunha, and J.-F. Chen (2013) Antagonistic interaction between adenosine A2A receptors and Na+/K+-ATPase-α2 controlling glutamate uptake in astrocytes. J. Neurosci. 33: 18492–18502.
Blaustein, M. P. and V. A. Golovina (2001) Structural complexity and functional diversity of endoplasmic reticulum Ca(2+) stores. Trends Neurosci. 24: 602–608.
Acknowledgements
This research was supported by Daegu University Research Grant, 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest associated with this study.
We used commercially available cell model and did not use any human or animal samples.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manigandan, S., Yun, J.W. Sodium-potassium Adenosine Triphosphatase α2 Subunit (ATP1A2) Negatively Regulates UCP1-dependent and UCP1-independent Thermogenesis in 3T3-L1 Adipocytes. Biotechnol Bioproc E 28, 644–657 (2023). https://doi.org/10.1007/s12257-023-0095-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-023-0095-3