Abstract
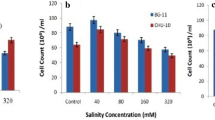
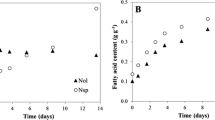
Salt stress can be used to increase intracellular lipid content of microalgae. However, the growth of microalgae is decreased under salt stress. Thus, a two-stage cultivation strategy with a growth phase followed by a stress stage is required to improve lipid content. In this study, adaptive laboratory evolution (ALE) with salt stress was conducted to improve biomass and fatty acid (FA) productivity using a locally isolated freshwater microalga Parachlorella sp. in a single-stage cultivation. Algal cell growth and FA production under conditions of salt stress (0–40 g of NaCl/L) were compared to those in a 0.5 L bubble column photobioreactor and appropriate levels of salt stress (10 and 20 g/L) were determined for ALE. During the ALE process, the average cell size increased from 3.0 to 3.9 µm. After eight consecutive ALE cycles, microalgal growth rate was remarkably increased to close to that of the culture without salt stress. Furthermore, FA content in microalga was improved from 7.5% to 25%. This result was confirmed by observations with various types of microscopes. Eventually, overall FA productivity was increased up to 219.0 ± 10.7 mg/L/day with the addition of 20 g of NaCl/L, which was 233% greater than that of the culture without salt stress (93.8 ± 5.3 mg/L/day) due to increased FA content. Recovered biomass productivity was 80% of that in the culture without salt stress. These results suggest that microalgal FA production can be significantly improved by a simple ALE process without an additional stress culture step.
Similar content being viewed by others
References
Ramanna, L., I. Rawat, and F. Bux (2017) Light enhancement strategies improve microalgal biomass productivity. Renew. Sust. Energy Rev. 80: 765–773.
Lee, H. S., Z. H. Kim, H. Park, and C. G. Lee (2016) Specific light uptake rates can enhance astaxanthin productivity in Haematococcus lacustris. Bioprocess Biosyst. Eng. 39: 815–823.
Begum, H., F. M. Yusoff, S. Banerjee, H. Khatoon, and M. Shariff (2016) Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 56: 2209–2222.
Kim, Z. H., Y. S. Park, Y. J. Ryu, and C. G. Lee (2017) Enhancing biomass and fatty acid productivity of Tetraselmis sp. in bubble column photobioreactors by modifying light quality using light filters. Biotechnol. Bioprocess Eng. 22: 397–404.
Zhang, T. Y., H. Y. Hu, Y. H. Wu, L. L. Zhuang, X. Q. Xu, X. X. Wang, and G. H. Dao (2016) Promising solutions to solve the bottlenecks in the large-scale cultivation of microalgae for biomass/bioenergy production. Renew. Sust. Energy Rev. 60: 1602–1614.
Salama, E. S., M. B. Kurade, R. A. I. Abou-Shanab, M. M. El-Dalatony, I. S. Yang, B. Min, and B. H. Jeon (2017) Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sust. Energy Rev. 79: 1189–1211.
Derakhshandeh, M. and U. T. Un (2019) Optimization of microalgae Scenedesmus sp. growth rate using a central composite design statistical approach. Biomass Bioeng. 122: 211–220.
Tran, H. L., J. S. Kwon, Z. H. Kim, Y. Oh, and C. G. Lee (2010) Statistical optimization of culture media for growth and lipid production of Botryococcus braunii LB572. Biotechnol. Bioprocess Eng. 15: 277–284.
Larkum, A. W. D., I. L. Ross, O. Kruse, and B. Hankamer (2012) Selection, breeding and engineering of microalgae for bioenergy and biofuel production. Trends Biotechnol. 30: 198–205.
Park, H., D. Jung, J. Lee, P. Kim, Y. Cho, I. Jung, Z. H. Kim, S. M. Lim, and C. G. Lee (2018) Improvement of biomass and fatty acid productivity in ocean cultivation of Tetraselmis sp. using hypersaline medium. J. Appl. Phycol. 30: 2725–2735.
Wang, Y., B. He, Z. Sun, and Y. F. Chen (2016) Chemically enhanced lipid production from microalgae under low suboptimal temperature. Algal Res. 16: 20–27.
Ra, C. H., C. H. Kang, N. K. Kim, C. G. Lee, and S. K. Kim (2015) Cultivation of four microalgae for biomass and oil production using a two-stage culture strategy with salt stress. Renew. Energy. 80: 117–122.
Kim, B. H., R. Ramanan, Z. Kang, D. H. Cho, H. M. Oh, and H. S. Kim (2016) Chlorella sorokiniana HS1, a novel freshwater green algal strain, grows and hyperaccumulates lipid droplets in seawater salinity. Biomass Bioenerg. 85: 300–305.
Li, Y., H. Xu, F. Han, J. Mu, D. Chen, B. Feng, and H. Zeng (2015) Regulation of lipid metabolism in the green microalga Chlorella protothecoides by heterotrophy-photoinduction cultivation regime. Bioresour. Technol. 192: 781–791.
Fu, W., O. Gudmundsson, A. M. Feist, G. Herjolfsson, S. Brynjolfsson, and B. Ø. Palsson (2012) Maximizing biomass productivity and cell density of Chlorella vulgaris by using light-emitting diode-based photobioreactor. J. Biotechnol. 161: 242–249.
Sun, X. M., L. J. Ren, Z. Q. Bi, X. J. Ji, Q. Y. Zhao, L. Jiang, and H. Huang (2018) Development of a cooperative two-factor adaptive-evolution method to enhance lipid production and prevent lipid peroxidation in Schizochytrium sp. Biotechnol. Biofuels. 11: 65.
Tran, H. L., S. J. Hong, and C. G. Lee (2009) Evaluation of extraction methods for recovery of fatty acids from Botryococcus braunii LB 572 and Synechocystis sp. PCC 6803. Biotechnol. Bioprocess Eng. 14: 187–192.
Spurr, A. R. (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26: 31–43.
Reynolds, E. S. (1963) Liver parenchymal cell injury: I. Initial alterations of the cell following poisoning with carbon tetrachloride. J. Cell Biol. 19: 139–157.
Kim, Z. H., H. Park, Y. J. Ryu, D. W. Shin, S. J. Hong, H. L. Tran, S. M. Lim, and C. G. Lee (2015) Algal biomass and biodiesel production by utilizing the nutrients dissolved in seawater using semi-permeable membrane photobioreactors. J. Appl. Phycol. 27: 1763–1773.
Talebi, A. F., M. Tabatabaei, S. K. Mohtashami, M. Tohidfar, and F. Moradi (2013) Comparative salt stress study on intracellular ion concentration in marine and salt-adapted freshwater strains of microalgae. Not. Sci. Biol. 5: 309–315.
Allakhverdiev, S. I., A. Sakamoto, Y. Nishiyama, M. Inaba, and N. Murata (2000) Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 123: 1047–1056.
Takagi, M., Karseno, and T. Yoshida (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 101: 223–226.
Fon-Sing, S. and M. A. Borowitzka (2016) Isolation and screening of euryhaline Tetraselmis spp. suitable for large-scale outdoor culture in hypersaline media for biofuels. J. Appl. Phycol. 28: 1–14.
Church, J., J. H. Hwang, K. T. Kim, R. McLean, Y. K. Oh, B. Nam, J. C. Joo, and W. H. Lee (2017) Effect of salt type and concentration on the growth and lipid content of Chlorella vulgaris in synthetic saline wastewater for biofuel production. Bioresour. Technol. 243: 147–153.
Klok, A. J., P. P. Lamers, D. E. Martens, R. B. Draaisma, and R. H. Wijffels (2014) Edible oils from microalgae: insights in TAG accumulation. Trends Biotechnol. 32: 521–528.
Wang, D., W. Wang, N. Xu, and X. Sun (2016) Changes in growth, carbon and nitrogen enzyme activity and mRNA accumulation in the halophilic microalga Dunaliella viridis in response to NaCl stress. J. Ocean Univ. China. 15: 1094–1100.
Takeshita, T., S. Ota, T. Yamazaki, A. Hirata, V. Zachleder, and S. Kawano (2014) Starch and lipid accumulation in eight strains of six Chlorella species under comparatively high light intensity and aeration culture conditions. Bioresour. Technol. 158: 127–134.
Mujtaba, G., W. Choi, C. G. Lee, and K. Lee (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour. Technol. 123: 279–283.
Acknowldgments
This study was supported by a grant (Project No. NNIBR202102104) from Nakdonggang National Institute of Biological Resources funded by the Ministry of Environment of the Korean government. This work was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1I1A1A 01042404). The authors would like to thank Ms. Ye Ji Park for experimental assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ethical Statements
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kim, ZH., Kim, K., Park, H. et al. Enhanced Fatty Acid Productivity by Parachlorella sp., a Freshwater Microalga, via Adaptive Laboratory Evolution Under Salt Stress. Biotechnol Bioproc E 26, 223–231 (2021). https://doi.org/10.1007/s12257-020-0001-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-020-0001-1